Method for preparing propylene and aromatic hydrocarbon from methyl alcohol or/and dimethyl ether
A technology of dimethyl ether and methanol, applied in chemical instruments and methods, producing hydrocarbons from oxygen-containing organic compounds, ethylene production, etc., can solve problems such as hindering the production of aromatics from methanol, reduced economic benefits, and low-yield aromatics, so as to avoid Effects of high cost, improved economy, and increased yield of aromatics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
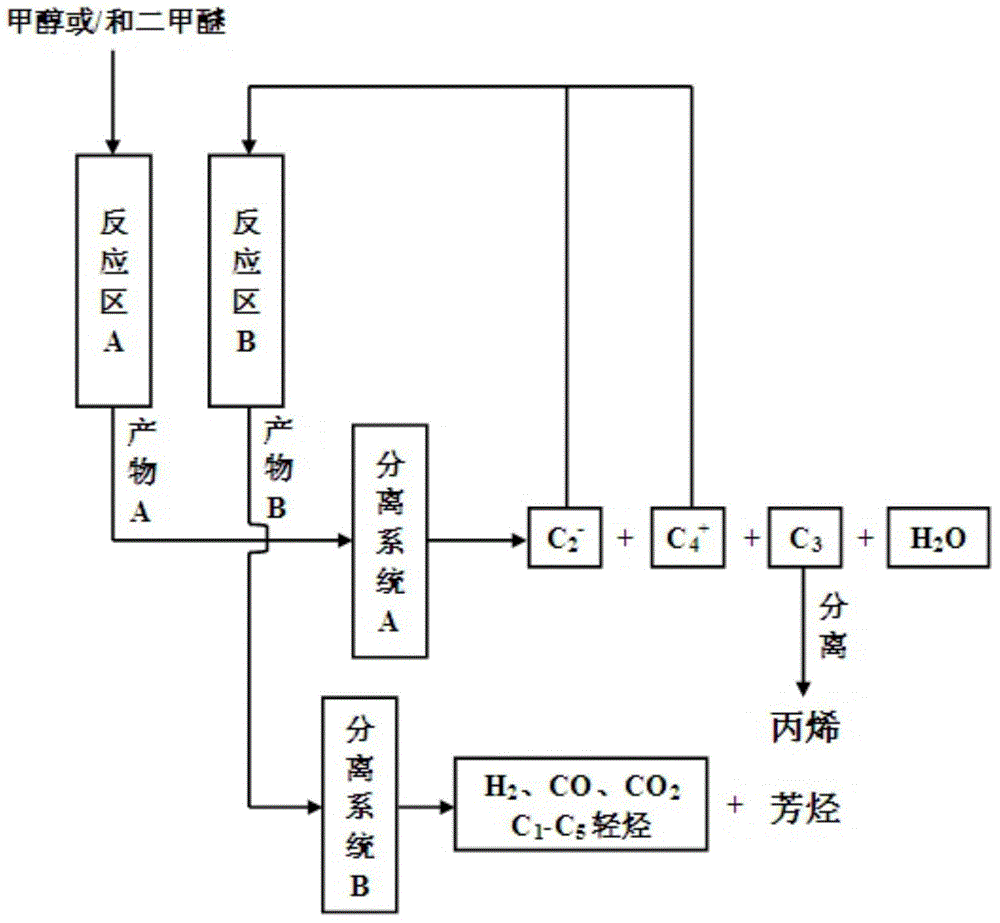
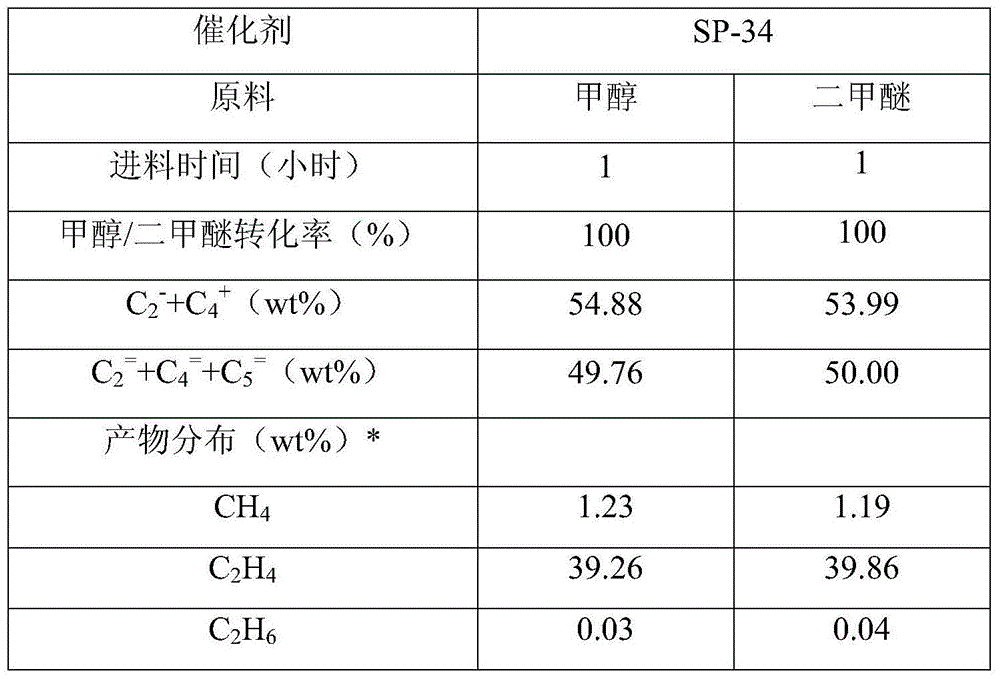
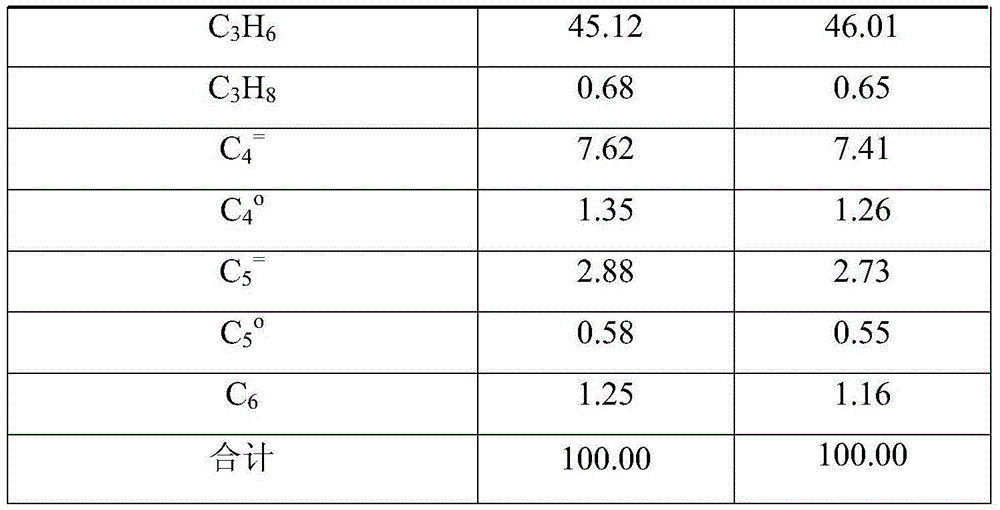
[0035] The SP-34 catalyst was pressed into tablets and crushed and sieved into 40-60 mesh catalyst samples, and 10 grams of the catalyst was loaded into the fixed-bed reactor in the reaction zone A to carry out the methanol or dimethyl ether conversion reaction respectively, methanol and dimethyl ether Mass space velocity is 2h -1 , the reaction temperature is 420°C. The composition of the product was analyzed online by gas chromatography, and the distribution of the product after removing the generated water is shown in Table 1.
[0036] The selectivity of propylene in the hydrocarbon products obtained by methanol or dimethyl ether conversion reaction on the SP-34 catalyst is 45.12wt%, 46.01wt% respectively; C 2 - and C 4 + The selectivities are 54.88wt%, 53.99wt%, respectively, where C 2 = +C 4 = +C 5 = Olefin selectivities were 49.76wt% and 50.00wt% respectively.
[0037] Table 1 Methanol / dimethyl ether produces light olefin reaction result
[0038]
[0039] ...
Embodiment 2
[0054] The SP-34 catalyst is pressed into tablets, crushed and screened into 40-60 mesh catalyst samples, and 10 grams of catalyst is loaded into the fixed-bed reactor in reaction zone A to carry out methanol or dimethyl ether conversion reaction, methanol or dimethyl ether Mass space velocity is 0.5h -1 , the reaction temperature is 350°C. Propylene selectivity in the hydrocarbon product that reaction obtains is respectively 47.28wt%, 48.23wt%; C 2 - and C 4 + The selectivities are 52.72wt%, 51.77wt%, respectively, where C 2 = +C 4 = +C 5 = Olefin selectivities were 47.61wt%, 47.92wt%, respectively.
[0055] The Ga-HZ-5 catalyst was pressed into tablets, crushed and sieved into a catalyst sample of 40-60 mesh, and 10 grams of the catalyst was loaded into a fixed reactor in the reaction zone B. The methanol conversion reaction product distribution in C 2 - components and C 4 + Composition, return to the reaction zone B of the reaction system and contact with the...
Embodiment 3
[0058] The SP-34 catalyst was pressed into tablets and crushed and sieved into 40-60 mesh catalyst samples, and 10 grams of the catalyst was loaded into the fixed-bed reactor in the reaction zone A to carry out the methanol or dimethyl ether conversion reaction respectively, methanol and dimethyl ether Mass space velocity is 8h -1 , the reaction temperature is 500°C. Propylene selectivity is respectively 42.65wt%, 43.14wt% in the hydrocarbon product that reaction obtains; C 2 - and C 4 + The selectivities are 57.35wt%, 56.86wt%, respectively, where C 2 = +C 4 = +C 5 = Olefin selectivities were 51.68wt%, 52.57wt%, respectively.
[0059] The Ga-HZ-5 catalyst was pressed into tablets, crushed and sieved into a catalyst sample of 40-60 mesh, and 10 grams of the catalyst was loaded into a fixed reactor in the reaction zone B. The methanol conversion reaction product distribution in C 2 - components and C 4 + Composition, return to the reaction zone B of the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com