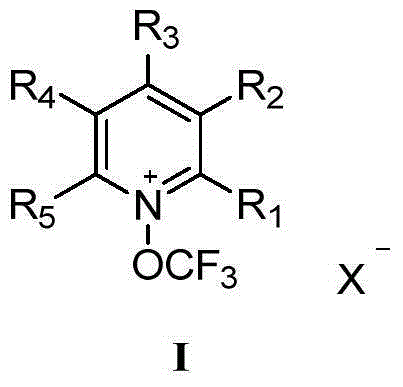
N- trifluoromethoxy pyridine salt compound and preparation method and use thereof
A compound and alkoxy technology, which is applied in ether preparation, organic chemistry, etc., can solve problems such as the lack of methods for trifluoromethoxylation reaction, harsh reaction conditions, and complicated steps of trifluoromethoxylation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
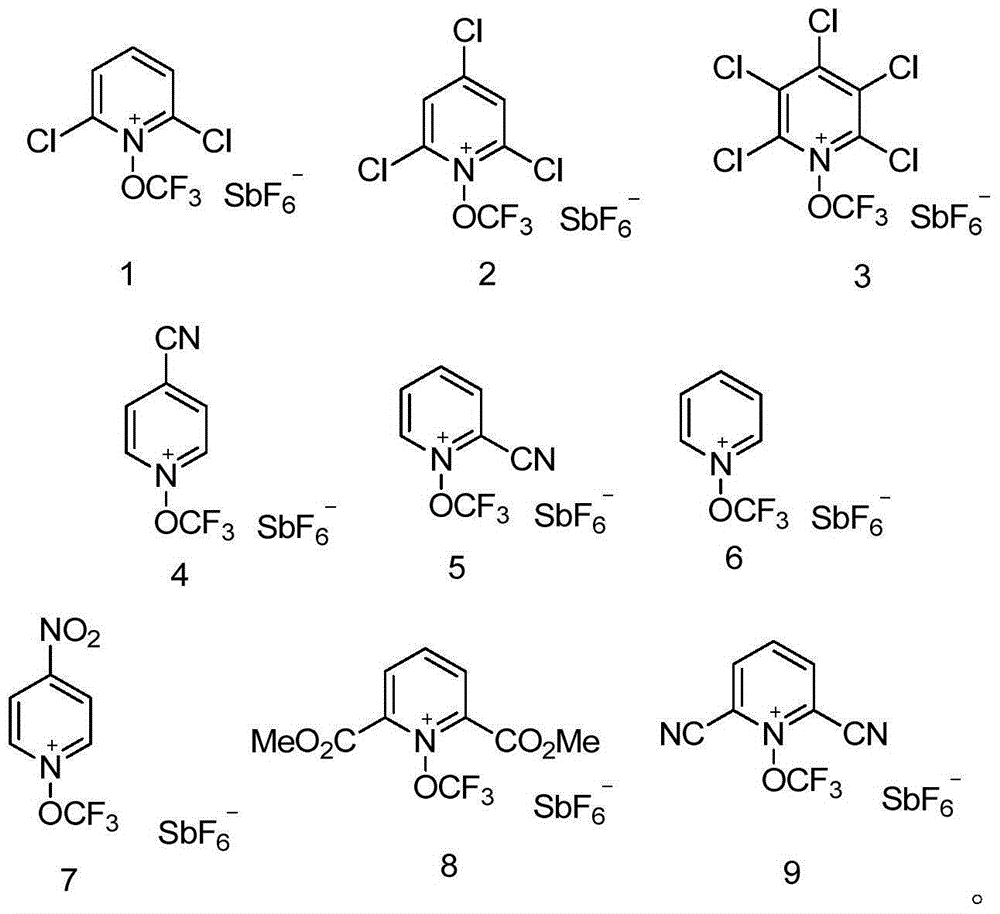
[0062] Synthesis of N-trifluoromethoxy-2,6-dichloropyridine hexafluoroantimonate
[0063]
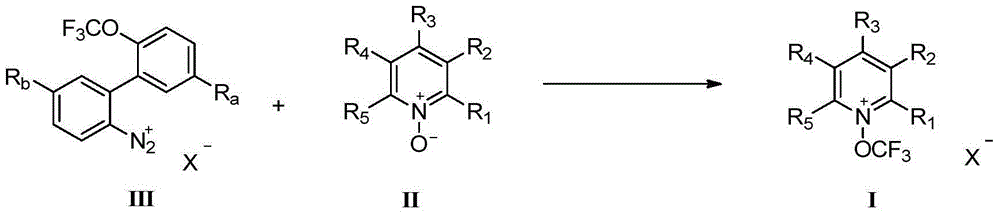
[0064] Under a nitrogen atmosphere, the compound 2-(trifluoromethoxy)biphenyl-2'-hexafluoroantimonate diazonium salt (3.63g, 7.26mmol) was added to a 100mL sealed tube, and 30mL of dichloromethane was added, 2,6 - Dichloropyridine nitroxide (2.38 g, 14.5 mmol), 3.5 hours at 42°C. The solid was precipitated by cooling, part of the solvent was evaporated under reduced pressure, allowed to stand, and filtered to obtain 1.73 g of a white solid with a yield of 51% and a nuclear magnetic purity greater than 97%. Further purification was obtained by recrystallization from THF / anhydrous ether. white solid. The melting point is 205-207°C. Infrared characterization (potassium bromide film): 3101, 1583, 1557, 1477, 1460, 1310, 1257, 1180, 1106, 904, 831, 780, 658cm -1 .NMR spectrum: 1 H NMR characterization (300MHz, CD 3 CN, 23℃, δ): 8.67(t, J=8.5Hz, 1H), 8.35(d, J=8.4Hz, 2H). 19 F nucle...
Embodiment 2
[0066] Synthesis of N-trifluoromethoxy-2,4,6-trichloropyridine hexafluoroantimonate
[0067]
[0068] Under a nitrogen atmosphere, add the compound 2-(trifluoromethoxy)biphenyl-2'-hexafluoroantimonate diazonium salt (1.25g, 2.5mmol) into a 25mL sealed tube, add 10mL of dichloromethane, 2,4 , 6-trichloropyridine nitroxide (496mg, 2.5mmol), at 42°C for 3 hours. The solid was precipitated by cooling, and part of the solvent was evaporated under reduced pressure, allowed to stand, and filtered to obtain 719 mg of a white solid with a yield of 57% and a nuclear magnetic purity greater than 97%. Further purification was obtained by recrystallization from THF / anhydrous ether. white solid. Melting point: 194-195°C. Infrared characterization (potassium bromide film): 3078, 1582, 1537, 1421, 1395, 1308, 1263, 1115, 876, 860, 793, 660cm -1 .NMR spectrum: 1 H NMR characterization (300MHz, CD 3 CN,23℃,δ):8.37(s). 19 F nuclear magnetic characterization (375MHz, CD 3 CN,23℃,δ):-59...
Embodiment 3
[0070] Synthesis of N-trifluoromethoxy-2,3,4,5,6-pentachloropyridine hexafluoroantimonate
[0071]
[0072] Under a nitrogen atmosphere, add the compound 2-(trifluoromethoxy)biphenyl-2'-hexafluoroantimonate diazonium salt (1.25g, 2.50mmol) into a 100mL sealed tube, add 10mL of dichloromethane, 2,3 , 4,5,6-pentachloropyridine nitroxide (668mg, 2.5mmol), at 42°C for 3.5 hours. The solid was precipitated by cooling, and part of the solvent was evaporated under reduced pressure, allowed to stand, and filtered to obtain 670 mg of a white solid with a yield of 47% and a nuclear magnetic purity greater than 97%. Further purification was obtained by recrystallization from THF / anhydrous ether. white solid. Infrared characterization (potassium bromide film): 1382, 1331, 1313, 1173, 1011, 765, 740cm -1 .NMR spectrum: 19 F nuclear magnetic characterization (375MHz, CD 3 CN,23℃,δ):-58.31(s),-111.01to-136.96(m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com