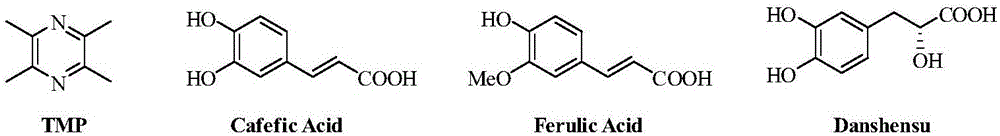
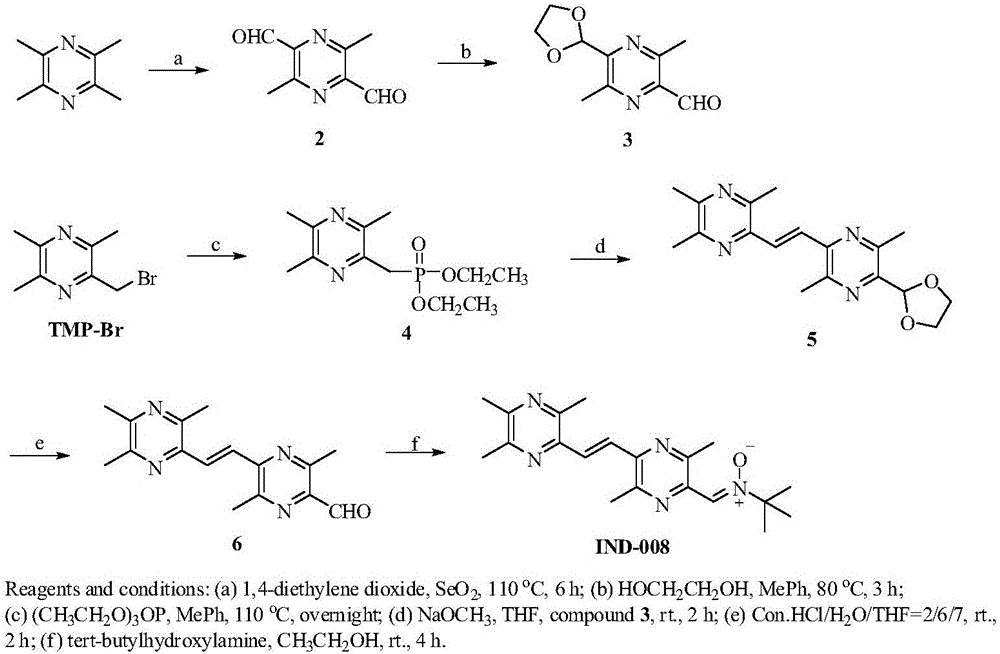
Novel pyrazine derivatives, preparation method therefor and medical application thereof
一种衍生物、吡嗪类的技术,应用在医药领域,能够解决氧化变质、需反复给药、半衰期短等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
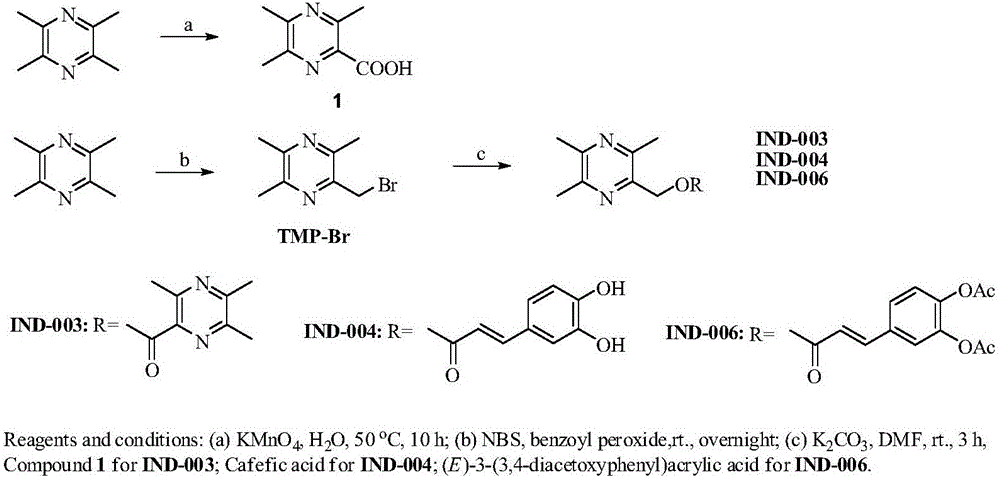
[0090] Embodiment 1, the synthesis of compound IND-003 ( figure 2 )
[0091] TMP (13.6g, 100.0mmol) was dissolved in 300mL of water, and KMnO was added in batches 4 (31.6g, 200.0mmol), heated to 50°C, reacted for 10h. After the reaction is complete, cool, extract with ethyl acetate, discard the organic phase, adjust the pH value of the aqueous phase to 3 with 10% hydrochloric acid, then extract with ethyl acetate, dry over anhydrous sodium sulfate, and evaporate the solvent under reduced pressure. 3,5,6-Trimethylpyrazine-2-carboxylic acid (Compound 1) (9.6 g, 57.8%) was obtained as a yellow solid. ESI-MS:[M+H] + m / z167.0.
[0092] TMP (15g, 110.3mmol) was dissolved in 250mL of carbon tetrachloride, NBS (20g, 112.4mmol) and catalytic amount of benzoyl peroxide were added respectively, heated to 80°C, and reacted overnight. After the reaction was complete, an appropriate amount of water was added, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and evapo...
Embodiment 2
[0094] Embodiment 2, the synthesis of compound IND-004 ( figure 2 )
[0095] Dissolve caffeic acid (0.36g, 2mmol) in 10mL of N,N-dimethylformamide, add K 2 CO 3 (0.31g, 2.4mmol), TMP-Br (0.43g, 2mmol), stirred at room temperature for 2h. After the reaction was complete, an appropriate amount of water was added, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. Column chromatography separation (ethyl acetate:petroleum ether=1:1) gave white solid compound IND-004 (0.36g, 57%). ESI-MS:[M+H] + m / z 315.23. 1 H-NMR: (300MHz, DMSO-d6) δ: 9.62(s, 1H), 9.16(s, 1H), 7.50(d, J=15.9Hz, 1H), 7.05(d, J=1.8Hz, 1H) ,7.01(dd,J=8.1,1.8Hz,1H),6.76(d,J=8.1Hz,1H),6.32(d,J=15.9Hz,1H),5.24(s,2H),2.48(s, 3H), 2.44(s,3H), 2.42(s,3H). 13 C-NMR: 166.72, 151.27, 149.06, 149.00, 148.90, 146.20, 146.03, 145.34, 125.90, 122.00, 116.18, 115.39, 113.83, 64.86, 21.69, 21.47, 20.58. Anal. (C 17 h 18 N 2 o 4 ) C, H, C; found C 64.92...
Embodiment 3
[0096] Embodiment 3, the synthesis of compound IND-006 ( figure 2 )
[0097] Dissolve (E)-3-(3,4-diacetoxyphenyl)acrylic acid (0.53g, 2mmol) in 10mL of N,N-dimethylformamide, add K 2 CO 3 (0.31g, 2.4mmol), TMP-Br (0.43g, 2mmol), stirred at room temperature for 2h. After the reaction was complete, an appropriate amount of water was added, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. Separation by column chromatography (ethyl acetate:petroleum ether=1:1) gave white solid compound IND-006 (0.60 g, 75%). ESI-MS:[M+H] + m / z 399.28. 1 H-NMR: (300MHz, CDCl 3 )δ: 7.65(d, J=15.9Hz, 1H), 7.40(dd, J=8.4, 2.1Hz, 1H), 7.35(d, J=2.1Hz, 1H), 7.21(d, J=8.4Hz, 1H), 6.43(d, J=15.9Hz, 1H), 5.53(s, 2H), 2.57(s, 3H), 2.52(s, 6H), 2.30(s, 6H). 13 C-NMR: 168.06, 167.98, 166.17, 151.43, 149.14, 144.72, 143.60, 143.57, 142.43, 133.12, 126.44, 123.96, 122.82, 118.58, 65.29, 21.70, 21.46, 20.66, 21 h 22 N 2 o 6 ) C, H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com