Recombinant pichia pastoris capable of expressing keratinase and application of recombinant pichia pastoris
A technology of keratinase and Pichia pastoris, which is applied in the field of genetic engineering, can solve the problems of product enzyme activity reduction, keratinase toxicity to host bacteria, and inability to industrialize production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Construction of embodiment 1 recombinant strain
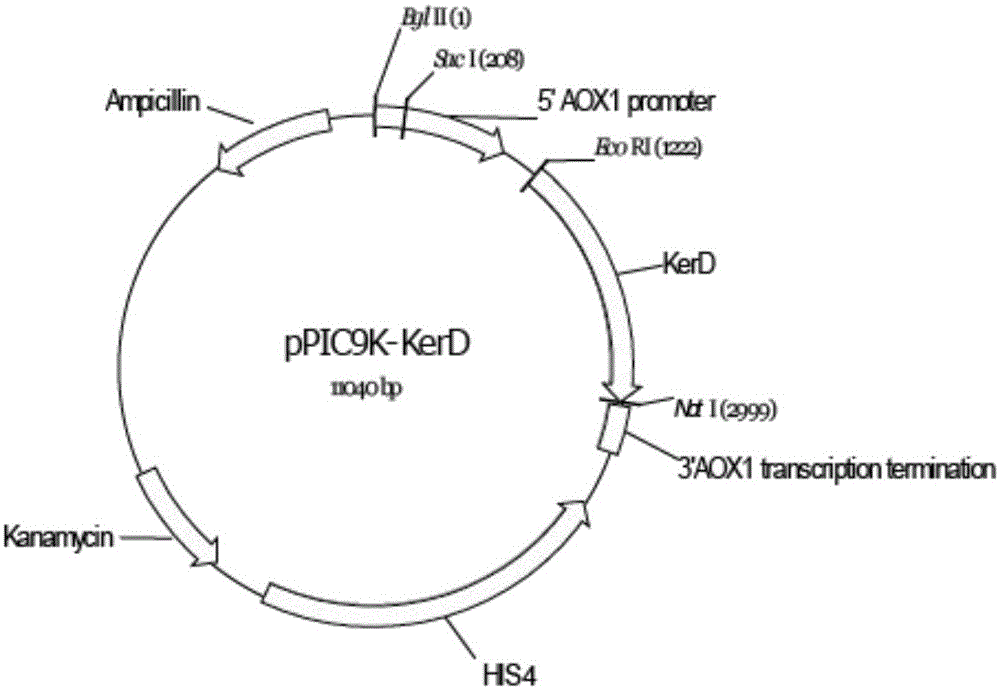
[0015] The gene encoding keratinase was cloned from Stenotrophomonas maltophilia BBE11-1, and after optimization, the gene encoding keratinase with nucleotide sequence as shown in SEQ ID NO.1 was obtained, and then cloned into expression plasmid pPIC9k to obtain recombinant plasmid pPIC9k -kerD. Afterwards, the recombinant plasmid was linearized and integrated into Pichia pastoris SMD1168 competent cells.
[0016] details as follows:
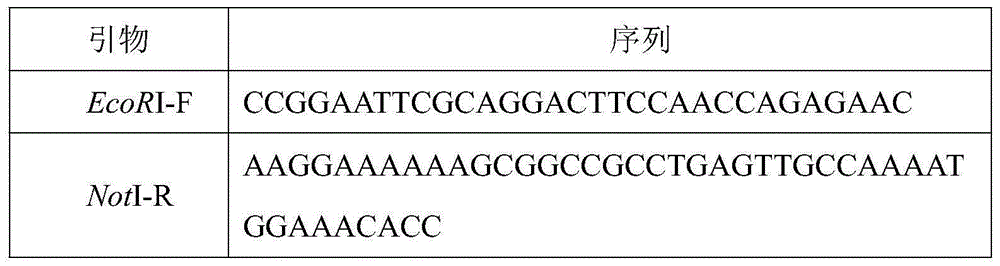
[0017] According to the gene sequence of kerD, primers EcoRI-F and NotI-R as shown in SEQ ID NO.2 and SEQ ID NO.3 were designed, and EcoRI and NotI restriction sites were added to both ends of the gene by PCR. The PCR product and the vector pPIC9k were digested with EcoRI and NotI respectively, and the digested products were purified and then ligated. The ligation product was transformed into Escherichia coli GM109 competent cells, positive clones were screened by LB plates containing k...
Embodiment 2
[0021] Induced expression and detection of embodiment 2keratinase
[0022] The recombinant strain was induced by methanol in a 250ml shake flask, and the keratinase activity of the supernatant of the fermentation broth was detected at different times, and the supernatant of the fermentation broth was analyzed by SDS-PAGE.
[0023] The induced expression of the recombinant strain is as follows:
[0024] 1) Transfer the recombinant strain to the YPD plate, then pick a single colony and inoculate it into a 250ml shake flask containing 25mL of BMGY medium, and culture it to OD at 28-30°C / 250-300rpm 600 =2-6(16-18h);
[0025] 2) Centrifuge at 1500-3000g for 5min at room temperature, collect the bacteria, and resuspend the bacteria with 50mL of BMMY medium to make the OD 600 = around 1.0;
[0026] 3) Put the bacterial liquid obtained in step 2 (about 100-200mL) into a 500mL shaker flask, seal it with double-layer gauze or cheesecloth, and place it on a shaker at 28-30°C / 250-300rp...
Embodiment 3
[0030] Verification on the enzyme production performance of embodiment 3 recombinant strains on the 3L fermenter
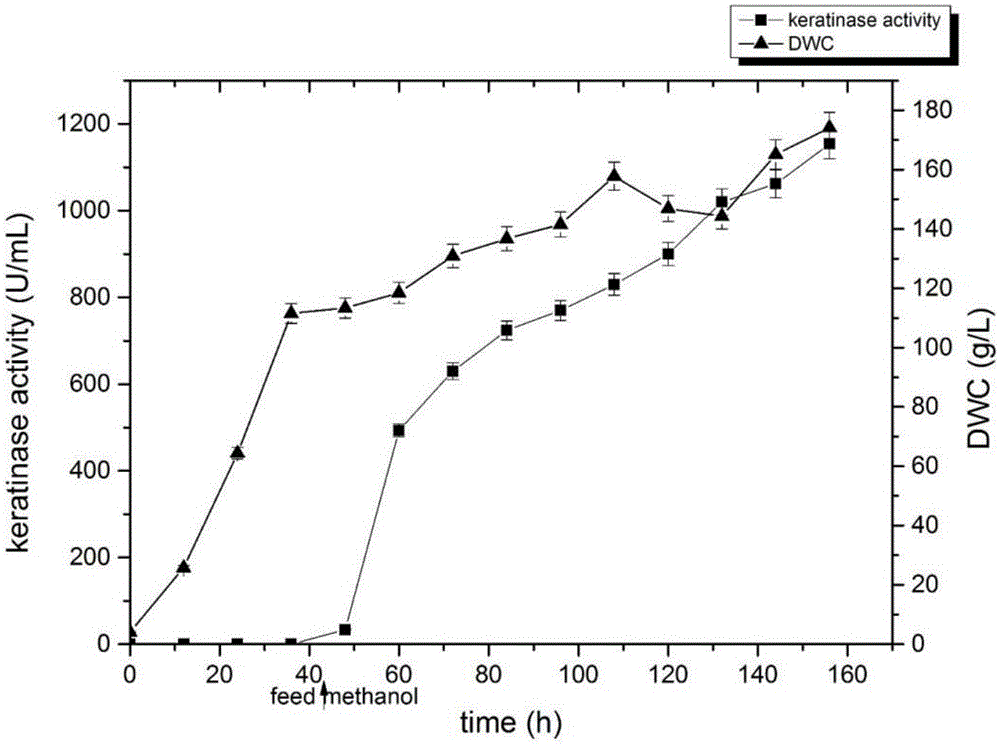
[0031] The recombinant strain was transferred to the YPD plate, and a single colony was picked and inoculated into a 1L shake flask containing 100 mL of YPD medium, and placed in a shaker at 30°C and 220 rpm for 24 hours as a seed solution. It was then inoculated into a 3L fully automatic fermenter (LiFlus GM BioTRON, Korea) containing 800 mL of BSM medium. Use 50% ammonia water and phosphoric acid solution to control pH5.5, temperature at 30°C, adjust the stirring speed to 500rpm, and maintain the ventilation at 2vvm; Add 350mL of 50% (W / V) glycerol (containing 12mL / L PTM1) to maintain the specific growth rate at 0.18h -1Left and right, and gradually adjust the stirring speed to 1000rpm, and the ventilation volume to 4vvm. After the glycerol is exhausted again, continue to maintain the matrix-deficient state in the system for about 1 hour and DO>60%, start feed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com