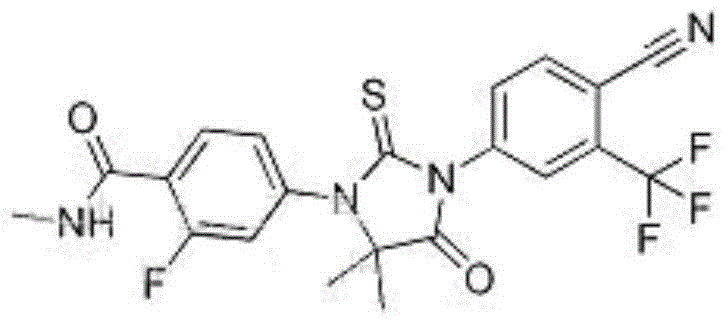
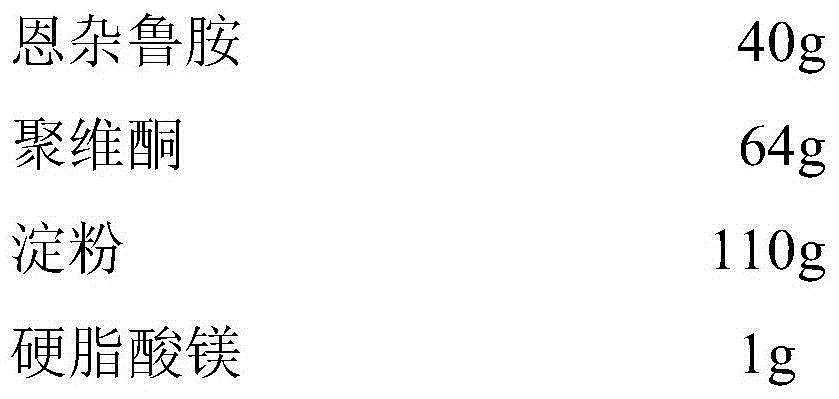Oral preparation of enzalutamide solid dispersion
A technology of solid dispersion and enzalutamide, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problem of complex soft capsule preparation process and low dissolution rate of preparations , Low bioavailability and other problems, to achieve the effect of improving bioavailability, improving dissolution rate, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1) Get ethyl cellulose, hydroxypropyl methyl cellulose, povidone K30, polyethylene glycol 6000, poloxamer each 60g respectively, dissolve in the ethanol aqueous solution that mass concentration is 60%, obtain the concentration of 20% water-soluble polymer carrier solution;
[0026] 2) Disperse 40g of enzalutamide in the above-mentioned five kinds of water-soluble polymer carrier solutions, stir and homogenize, then spray dry, control the inlet air temperature to 150°C, the outlet air temperature to 60°C, and the feed rate to be 10mL / min, spray pressure is 0.3MPa, obtain enzalutamide solid dispersion;
[0027] 3) Add 80g of lactose (filler), 10g of croscarmellose sodium (disintegrant), and 2g of magnesium stearate (lubricant) to each of the five enzalutamide solid dispersions, and mix well Tablet.
[0028] 1. Dissolution Determination
[0029] Take the tablet prepared in the above example 1 and 6 capsules of the original enzalutamide soft capsule, according to the dis...
Embodiment 2
[0044] Enzalutamide tablets, made from the following raw materials:
[0045]
[0046] The preparation method is:
[0047] 1) dissolving povidone K30 in 50% ethanol aqueous solution to obtain a water-soluble polymer carrier solution with a mass concentration of 15%;
[0048] 2) Disperse enzalutamide in the water-soluble polymer carrier solution, stir and homogenize, then spray dry. The air pressure is 0.1MPa to obtain a solid dispersion of enzalutamide;
[0049] 3) Add microcrystalline cellulose, croscarmellose sodium, and magnesium stearate to the solid dispersion of enzalutamide, mix well and then compress into tablets.
[0050] Each tablet of this product contains 40mg of enzalutamide, and the dosage is 1 tablet each time, once a day.
Embodiment 3
[0052] Enzalutamide capsules, made from the following ingredients:
[0053]
[0054] The preparation method is:
[0055] 1) dissolving povidone in 70% ethanol aqueous solution to obtain a water-soluble polymer carrier solution with a mass concentration of 30%;
[0056]2) Disperse enzalutamide in the water-soluble polymer carrier solution, stir and homogenize, then spray dry, control the inlet air temperature to 160°C, the outlet air temperature to 70°C, the feed rate to 20mL / min, Air pressure is 0.5MPa, obtains enzalutamide solid dispersion;
[0057] 3) Add starch and magnesium stearate to the enzalutamide solid dispersion, mix well and fill with capsules to make capsules.
[0058] Each capsule of this product contains 40mg of enzalutamide, and the dosage is 1 tablet each time, once a day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com