Method for splitting ofloxacin chiral drug through liquid-liquid-solid extraction
A technology of ofloxacin and chiral drugs, applied in the field of chiral drug separation, can solve the problems of complex operation process, high equipment cost, low efficiency, etc., and achieves a high separation selectivity, simple equipment and high extraction efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the concentration of ofloxacin aqueous solution is 1.0g / L extraction experiment
[0019] A. prepare 1.0g / L aqueous solution of ofloxacin, and the organic phase is n-hexane solution. Weigh 0.05375g D-DBTA (dibenzoyl tartaric acid) into a 50mL Erlenmeyer flask, then transfer 20mL each of the organic phase and the aqueous phase into the Erlenmeyer flask with a pipette. Seal the Erlenmeyer flask and place it in a THZ-82A digital display water bath constant temperature oscillator, shake at a speed of 220r / min for 40min, then place it in a centrifuge tube and centrifuge at a speed of 5000r / min for 10min in a centrifuge. The lower layer was filtered, concentrated, dried and purified to obtain ofloxacin crystals.
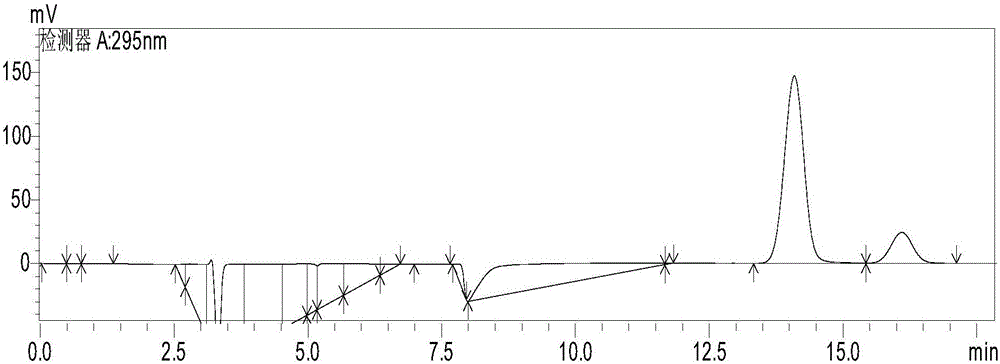
[0020] Use a disposable sterile syringe to draw 1ml of the aqueous phase clear liquid separated by centrifugation, filter through a 0.45μm filter head, inject 10μL into the high-performance liquid chromatography, and see the results in figure 1 ,Depen...
Embodiment 2
[0021] Embodiment 2: the extraction experiment that the concentration of ofloxacin feed liquid is 0.6g / L
[0022] Prepare 0.6g / L aqueous solution of ofloxacin as the water phase, and the organic phase as n-hexane solution. Weigh 0.0538g of D-DBTA, put it into a 50mL Erlenmeyer flask, transfer 20ml of n-hexane solution and 20mL ofloxacin aqueous solution with a concentration of 0.6g / L into the Erlenmeyer flask, seal the Erlenmeyer flask and place it in a digital display water bath for constant temperature The shaker THZ-82A oscillated at a speed of 220r / min for 40min, then placed in a centrifuge tube and centrifuged at a speed of 5000r / min for 10min in a centrifuge. The lower layer was filtered, concentrated, dried and purified to obtain ofloxacin crystals.
[0023] The aqueous phase clear liquid was analyzed by the same method as in Example 1, and the obtained separation factor was 3.052, and the ee value was 47.59%.
Embodiment 3
[0024] Embodiment 3: the extraction experiment that the concentration of ofloxacin feed liquid is 0.8g / L
[0025] Prepare 0.8g / L aqueous solution of ofloxacin as the water phase, and the organic phase as n-hexane solution. Weigh 0.0538g of D-DBTA, put it into a 50mL Erlenmeyer flask, transfer 20mL of n-hexane solution and 20mL ofloxacin aqueous solution with a concentration of 0.8g / L into the Erlenmeyer flask. Each Erlenmeyer flask was sealed and placed in a digital display water bath constant temperature oscillator THZ-82A, oscillating at a speed of 220r / min for 40min, then placed in a centrifuge tube and centrifuged at a speed of 5000r / min for 10min in a centrifuge. The lower layer was filtered, concentrated, dried and purified to obtain ofloxacin crystals.
[0026] Adopt the method with embodiment 1 to analyze aqueous phase clear liquid, experimental result shows that when feed liquid concentration is 0.8g / L, separation factor 4.175, ee value is 59.27%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com