Method for leaching solid arsenic out of arsenic sulfide slag through one step and enriching valuable metal
A valuable metal, arsenic sulfide technology, applied in the direction of improving process efficiency, can solve the problems of unindustrial application, unclear arsenic open circuit scheme, long process and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
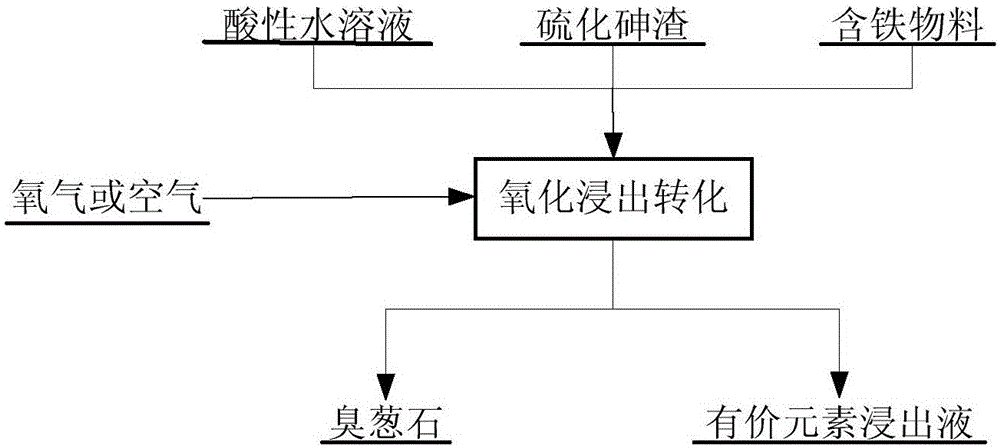
[0038] The main components of arsenic sulfide slag are (wt%): As19.17, Cu10.90, S19.25. Weigh ferrous sulfate (FeSO 4 .7H 2O), after mixing it with arsenic sulfide slag, use sulfuric acid aqueous solution to slurry, control the pH value of the slurry to 1.5, and the liquid-solid mass ratio to be 5, and place it in an autoclave; react at a temperature of 150°C and an oxygen pressure of 1.5MPa 3h; after the reaction, liquid-solid separation was achieved by suction filtration. 95% of the copper in the arsenic sulfide slag is dissolved in the leaching solution with copper sulfate, which can be further recovered; the arsenic sulfide in the arsenic sulfide slag is completely dissolved, and 95% of the arsenic is directly leached and transformed into crystalline scorodite into the slag, and the rest enters the In the leach solution; the leach toxicity of the leach residue mainly containing scorodite complies with the provisions of GB5085.3-2007 (Identification Standard for Solid Was...
Embodiment 2
[0040] The leaching slurry was prepared according to Example 1, placed in an autoclave, and reacted for 2 hours at 200° C. and an oxygen pressure of 1.0 MPa. After the reaction, liquid-solid separation was achieved by suction filtration. 98% of the copper in the arsenic sulfide slag is dissolved in the leaching solution with copper sulfate, which can be further recovered; the arsenic sulfide in the arsenic sulfide slag is completely dissolved, and 97% of the arsenic is directly leached and transformed into crystalline scorodite into the slag, and the rest enters the In the leach solution; the leach toxicity of the leach residue mainly containing scorodite complies with the provisions of GB5085.3-2007 (Identification Standard for Solid Waste - Identification of Leach Toxicity), and can be stored safely and stably; Return to configure leachate.
Embodiment 3
[0042] The leaching slurry was prepared according to Example 1, placed in an autoclave, and reacted for 2.5 hours at 250° C. and an oxygen pressure of 0.5 MPa. After the reaction, liquid-solid separation was achieved by suction filtration. 99% of the copper in the arsenic sulfide slag is dissolved in the leaching solution with copper sulfate, which can be further recovered; the arsenic sulfide in the arsenic sulfide slag is completely dissolved, and 98% of the arsenic is directly leached into crystalline scorodite and enters the slag, and the rest enters In the leach solution; the leach toxicity of the leach residue mainly containing scorodite complies with the provisions of GB5085.3-2007 (Identification Standard for Solid Waste - Identification of Leach Toxicity), and can be stored safely and stably; Return to configure leachate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com