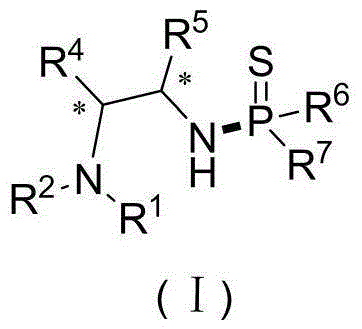
Application of beta-thioxophosphamide-containing amine compound
A technology of thiophosphoramide and compound is applied in the application field of resistance to tobacco mosaic virus disease and achieves the effect of good bactericidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
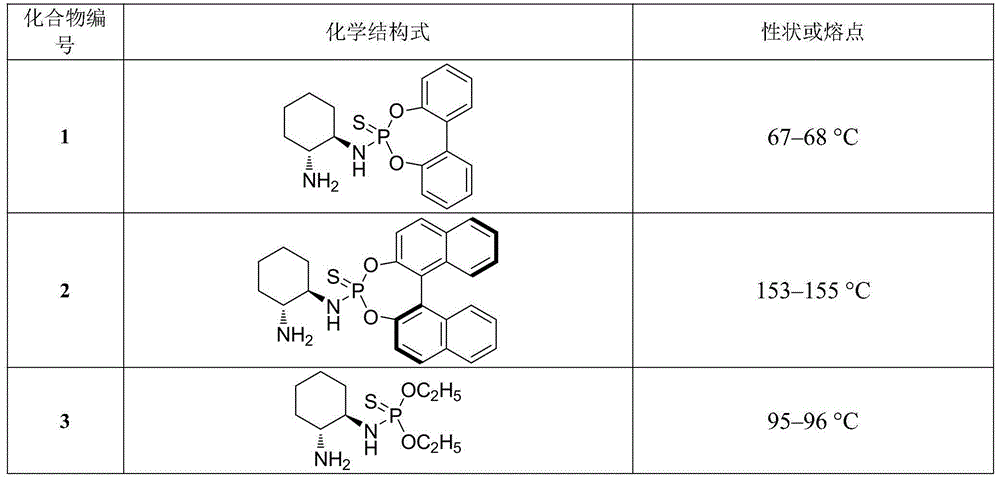
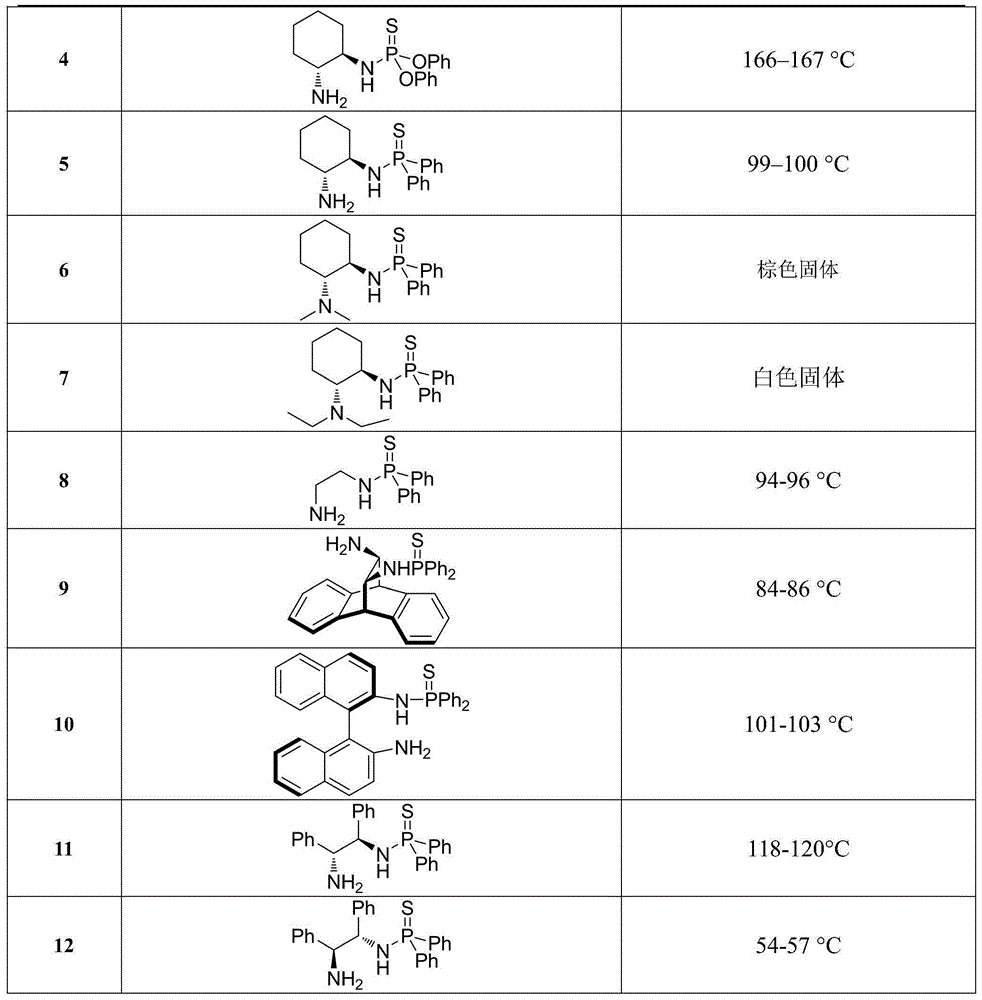
[0020] Example 1. The chemical structural formulas and physical constants of some β-thiophosphoramidoamine compounds are shown in Table 1:
[0021] Table 1. Some chemical structural formulas and physical constants of β-thiophosphoramidoamine compounds
[0022]
[0023]
Embodiment 2
[0024] Example 2: Preparation of target compound N-(1R,2R)-(1,2-diphenyl-2-amino)-P,P-diphenylthiophosphoramide (11)
[0025] At 0°C, (1R,2R)-1,2-diphenylethylenediamine (2.12g, 0.01mmol) was dissolved in dichloromethane (10mL), triethylamine (1.4mL) was added, and the reaction Stir in a container, then dissolve disubstituted thiophosphoryl chloride (0.252g, 0.01mmol) in dichloromethane (30mL), add dropwise to the reaction system with a constant pressure dropping funnel, and then TLC detection, until the raw material diphenyl After the disappearance of thiophosphoryl chloride, the product was precipitated and purified by column chromatography, and the obtained product was a white solid. The yield was 81%, and the melting point was 118-120°C. [α] 2 D 0 –23.4 (c1.0, CHCl 3 ); 1 HNMR (CDCl 3 ,400MHz): δ1.80(s,2H),4.19(t,J=6.8Hz,1H),4.25(d,J=5.6Hz,1H),4.44-4.51(m,1H),7.05-7.08( m,2H),7.11-7.15(m,3H),7.18-7.22(m,2H),7.24-7.38(m,9H),7.58-7.65(m,4H); 31 PNMR (CDCl 3 ,161.7M...
Embodiment 3
[0026] Embodiment 3: Determination of anti-tobacco mosaic virus activity of β-thiophosphoramidoamine-containing compounds, the determination procedure is as follows:
[0027] (1) Measurement method
[0028] 1.1. Virus purification and concentration determination:
[0029] Virus purification and concentration determination were carried out in accordance with the SOP specification for tobacco mosaic virus compiled by the Bioassay Laboratory of the Institute of Elements, Nankai University. After the crude virus extract was centrifuged twice with polyethylene glycol, the concentration was measured and refrigerated at 4°C for later use.
[0030] 1.2. Compound solution preparation:
[0031] After weighing, the original drug was dissolved in DMF to prepare 1×10 5 μg / mL mother solution, and then diluted with 1‰ Tween 80 aqueous solution to the required concentration; Ningnanmycin preparation was directly diluted with water.
[0032] 1.3. In vitro effect:
[0033] Rub inoculation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com