Anti-infective medicinal cefazedone sodium sterile composition
A technology of cefazedone sodium and its composition, which is applied in the field of anti-infective drug cefazedone sodium composition, can solve the problems of affecting the safety of the drug, high content of related substances, high content of substances, etc., and achieve improved stability and good fluidity , good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of Cefoxizone Sodium Crystals
[0028] (1) Take cefazedone sodium raw material and add it to deionized water, the volume of deionized water used is 3 times the mass of cefazedone sodium;
[0029] (2) Stir until completely dissolved, and adjust the pH to 6-7;
[0030] (3) Add activated carbon for decolorization and filter to obtain a clear solution;
[0031] (4) Move the clarified solution into the pressure vessel, add dropwise the acetone solution at 10°C under the condition of controlling the pressure in the pressure vessel at 1.5Mpa and stirring, the stirring speed is controlled at 30rmp, and the volume of the acetone solution is deionized water 5 times the volume of
[0032] (5) Release the pressure after the dropwise addition, cool the solution to -5°C at a rate of 5°C / min, let it stand for 2 hours, filter, wash with ether, and dry under reduced pressure to obtain crystals of cefazedone sodium.
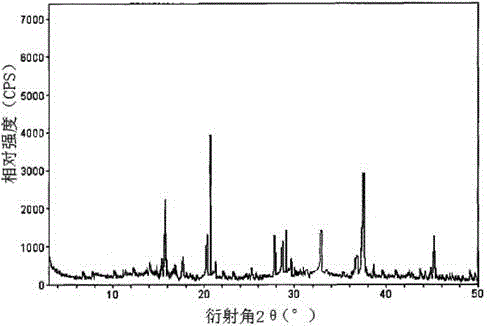
[0033] The X-ray powder diffraction patter...
Embodiment 2
[0034] Example 2: Preparation of Cefazedone Sodium Composition
[0035] The composition comprises: 1 part by weight of cefazedone sodium crystal prepared by the present invention, and 0.1 part by weight of sodium chloride.
[0036] The preparation method is:
[0037] (1) Weigh cefazedone sodium crystals and sodium chloride in proportion and mix them thoroughly;
[0038] (2) Dispense into sterilized vials and stopper them.
Embodiment 3
[0039] Example 3: Preparation of Cefazedone Sodium Composition
[0040] The composition comprises: 1 part by weight of cefazedone sodium crystal prepared by the present invention, and 0.2 part by weight of sodium chloride.
[0041] The preparation method is:
[0042] (1) Weigh cefazedone sodium crystals and sodium chloride in proportion and mix them thoroughly;
[0043] (2) Dispense into sterilized vials and stopper them.
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com