Anti-infective medicinal cefotiam hydrochloride composition
A technology of cefotiam hydrochloride and composition, which is applied in the field of anti-infective drug cefotiam hydrochloride composition, and can solve problems such as loss of antibacterial activity, poor mixing uniformity, unstable β-lactam ring, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of Cefotiam Hydrochloride Crystals
[0025] Take cefotiam hydrochloride raw material, add in the mixed solvent A of water and N-methylacetamide whose volume is 8 times of the weight of cefotiam hydrochloride at 30°C, the volume ratio of water and N-methylacetamide is 4:1 , to obtain the solution; then on the horizontal direction of the liquid surface of the obtained solution, the applied magnetic field intensity is a constant magnetic field of 0.5T, and under the condition of the constant magnetic field, the solution is dripped with a volume that is 5 times of the weight of cefotiam hydrochloride ethanol, iso Mixed solvent B of butanol and diethyl ether, the volume ratio of ethanol, isobutanol and diethyl ether is 2:3:4; after the dropwise addition is completed, cool down to -3°C, let stand for 2 hours, filter, wash, and vacuum dry to obtain Described cefotiam hydrochloride crystal.
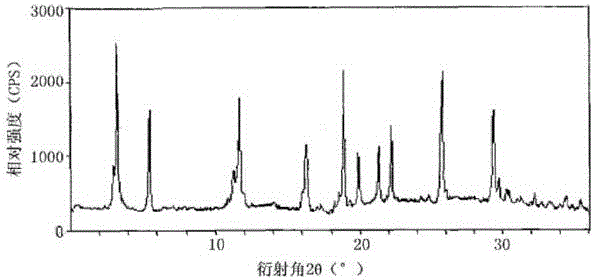
[0026] The prepared cefotiam hydrochloride crystal uses Cu-K...
Embodiment 2
[0027] Example 2: Preparation of cefotiam hydrochloride composition
[0028] The composition comprises: 1 part by weight of cefotiam hydrochloride crystal prepared by the invention, and 0.4 part by weight of sodium chloride.
[0029] The preparation method is:
[0030] (1) Weigh cefotiam hydrochloride crystals and sodium chloride in proportion and mix them thoroughly;
[0031] (2) Dispense into sterilized vials and stopper them.
Embodiment 3
[0032] Example 3: Preparation of cefotiam hydrochloride composition
[0033] The composition comprises: 1 part by weight of cefotiam hydrochloride crystal prepared by the invention, and 0.5 part by weight of sodium chloride.
[0034] The preparation method is:
[0035] (1) Weigh cefotiam hydrochloride crystals and sodium chloride in proportion and mix them thoroughly;
[0036] (2) Dispense into sterilized vials and stopper them.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com