Method for efficiently preparing quinazolinone derivants under promotion of ethyl alcohol and catalysis of titanocene dichloride
A dichloro-titanocene catalysis and dichloro-titanocene technology, which is applied in organic chemistry and other fields, achieves the effects of short time, mild reaction conditions and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
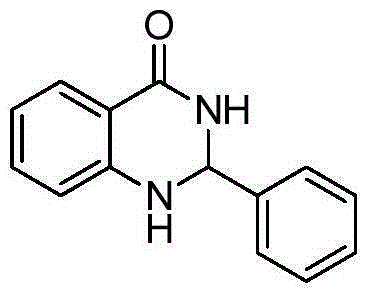
[0012] Taking the preparation of 2-phenyl-2,3-dihydroquinazolin-4(1H)-one as an example, the raw materials used and the preparation method are as follows:
[0013]
[0014] Add 0.0025g (0.01mmol) titanocene dichloride, 0.1362g (1mmol) anthranilamide, 102μL (1mmol) benzaldehyde, 0.2mL ethanol to a 10mL Shrek tube, and stir the reaction at 40°C for 10 minutes to stop the reaction. Add 10-15 mL of ethyl acetate, remove ethyl acetate by rotary evaporation, separate and remove the catalyst with a silica gel column (ethyl acetate: petroleum ether = 1:1), and recrystallize with 95% ethanol aqueous solution to obtain 2- The yield of phenyl-2,3-dihydroquinazolin-4(1H)-one is 96%. The spectral data of the product is: 1 HNMR(400MHz, DMSO-d 6 )δ: 8.30 (s, 1H), 7.64 (d, J = 7.6 Hz, 1H), 7.51 (d, J = 7.2 Hz, 2H), 7.40 (s, 3H), 7.30-7.20 (m, 1H), 7.12(s, 1H), 6.77(d, J=8.1Hz, 1H), 6.69(s, 1H), 5.77(s, 1H); 13 CNMR(101MHz, DMSO-d 6 ) δ: 165.50, 149.76, 143.52, 135.20, 130.34, 130.21, 129.25, 128...
Embodiment 2
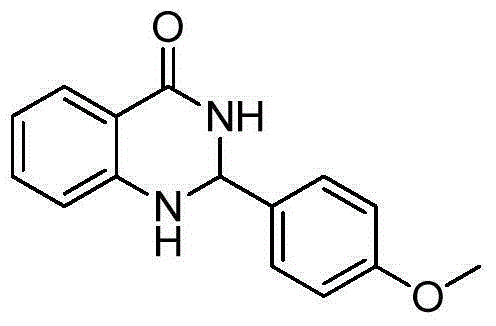
[0020] Taking the preparation of the compound 2-(4'-methoxyphenyl)-2,3-dihydroquinazolin-4(1H)-one as an example, the raw materials used and the preparation method are as follows:
[0021]
[0022] In Example 1, the benzaldehyde used was replaced with an equimolar 4-methoxybenzaldehyde, and the other steps were the same as in Example 1, to obtain 2-(4-methoxyphenyl)-2,3-dihydro The yield of quinazolin-4(1H)-one is 97%. The spectral data of the product is: 1 HNMR(400MHz, DMSO-d 6 )δ: 8.19(s, 1H), 7.62(d, J=7.6Hz, 1H), 7.43(d, J=8.6Hz, 2H), 7.25(s, 1H), 7.02(s, 1H), 6.96( s, 2H), 6.75(d, J=8.1Hz, 1H), 6.68(s, 1H), 5.72(s, 1H), 3.75(s, 3H); 13 CNMR(101MHz, DMSO-d 6 ) δ: 165.59, 161.32, 149.90, 135.36, 135.12, 130.10, 129.23, 118.97, 116.89, 116.30, 115.52, 68.20, 57.06.
Embodiment 3
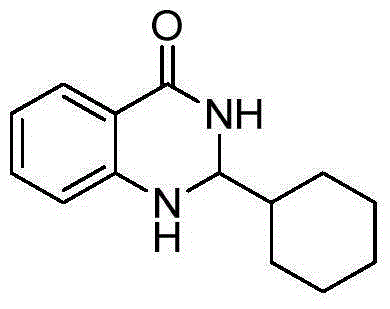
[0024] Taking the preparation of the compound 2-cyclohexane-2,3-dihydroquinazolin-4(1H)-one of the following formula as an example, the raw materials used and the preparation method are as follows:
[0025]
[0026] In Example 1, the benzaldehyde used was replaced with an equimolar cyclohexylformaldehyde, and the other steps were the same as in Example 1, to obtain 2-cyclohexane-2,3-dihydroquinazolin-4(1H)-one , The yield is 95%, and the spectrum data of the product is: 1 HNMR(400MHz, DMSO-d 6 )δ: 7.87(s, 1H), 7.55(d, J=7.6Hz, 1H), 7.19(s, 1H), 6.74(d, J=8.1Hz, 1H), 6.60(s, 1H), 6.54( s, 1H), 4.44 (s, 1H), 1.70 (d, J=10.6 Hz, 6H), 1.11 (s, 5H); 13 CNMR(101MHz, DMSO-d 6 ) δ: 165.58, 150.24, 134.90, 129.11, 118.30, 116.68, 115.96, 70.45, 44.74, 41.63, 41.42, 41.21, 28.88, 28.56, 27.82, 27.50, 27.44.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com