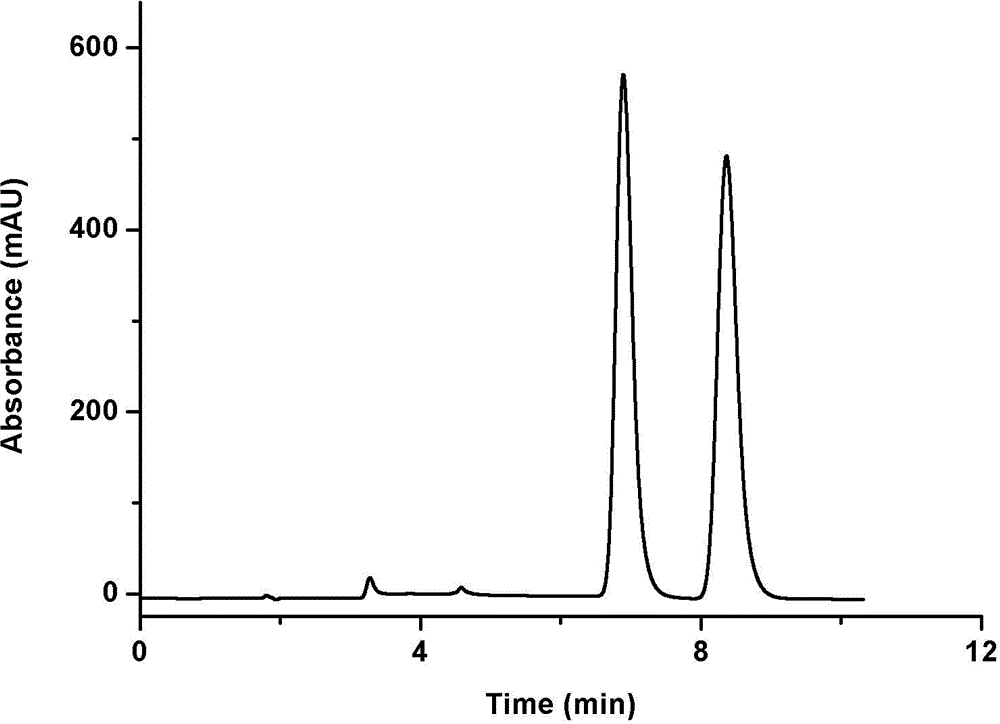
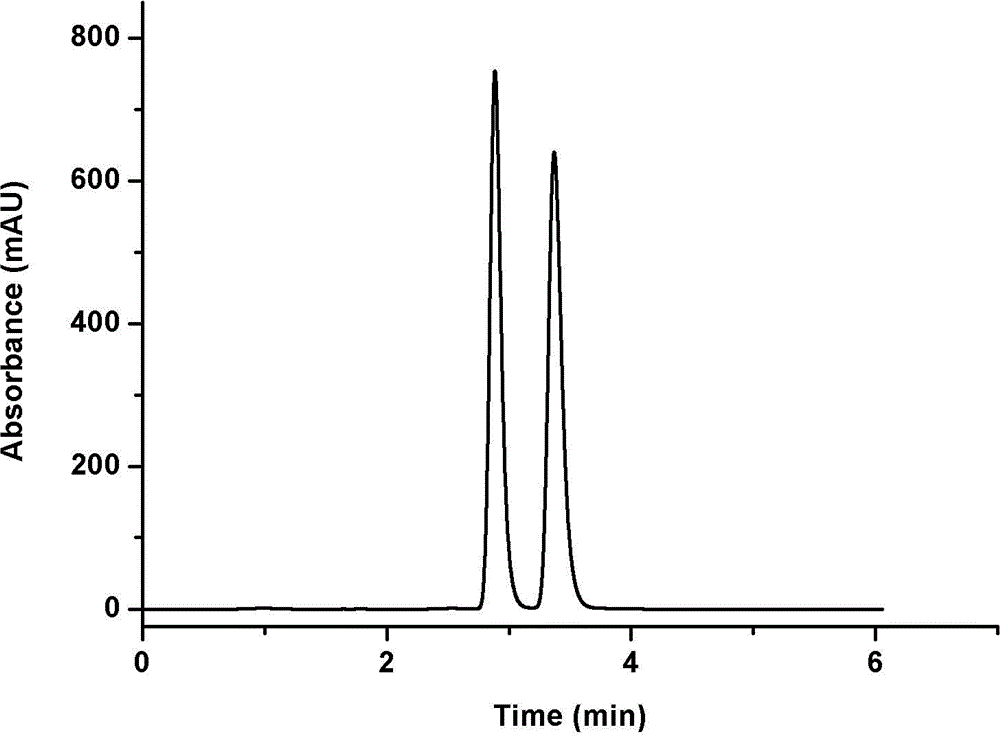
Chemically-bonded cellulose-type chiral stationary phase and preparation method thereof
A chiral stationary phase and cellulose-based technology, applied in chemical instruments and methods, and other chemical processes, can solve the problems of high pressure resistance, short service life, etc., and achieve good reproducibility, wide application range, and application range wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Activate 3g of silica gel and dry it overnight at 120°C, put it in a 100ml three-necked round-bottomed flask, add 30ml of dry toluene and 5ml of triethoxyaminopropylsilane in sequence, and heat it under nitrogen protection at 110°C for 24h. Filtrate while hot, wash, and dry overnight at 80°C to obtain aminopropyl silica gel.
[0021] (2) Preparation of cellulose derivatives
[0022] Put 1g of cellulose in a three-necked round-bottomed flask filled with 40ml of pyridine, add 3g of triphenylchloromethane and react at 100°C for 24h, then add 3g of 4-chloromethylphenyl isocyanate and continue to react at 100°C for 24h, cool Afterwards, the product was poured into 100 ml of methanol solution of hydrochloric acid, stirred at room temperature for 24 hours, filtered, washed with methanol, and dried in vacuum to obtain cellulose derivatives.
[0023] (3) chemical bonding
[0024] Dissolve 0.5g of cellulose derivatives in a beaker containing 30ml of tetrahydrofuran, add 2g ...
Embodiment 2
[0026] (1) Activate 15g of silica gel and dry it overnight at 120°C, place it in a 250ml three-neck round-bottomed flask, add 150ml of dry toluene, 25ml of triethoxyaminopropylsilane in turn, and reflux at 110°C for 24 hours under nitrogen protection. Filtrate while hot, wash, and dry overnight at 80°C to obtain aminopropyl silica gel.
[0027] (2) Preparation of cellulose derivatives
[0028] Put 3g of cellulose in a three-necked round-bottom flask filled with 100ml of pyridine, add 10g of triphenylchloromethane and react at 100°C for 24h, then add 10g of 4-chloromethylphenylisocyanate and continue to react at 100°C for 24h, cool Finally, the product was poured into 300ml of methanol solution of hydrochloric acid, and reacted for 24 hours, filtered, washed with methanol, and dried in vacuum to obtain cellulose derivatives.
[0029] (3) chemical bonding
[0030] Take 3g of cellulose derivatives and dissolve them in a beaker containing 180ml of tetrahydrofuran, add 12g of ami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com