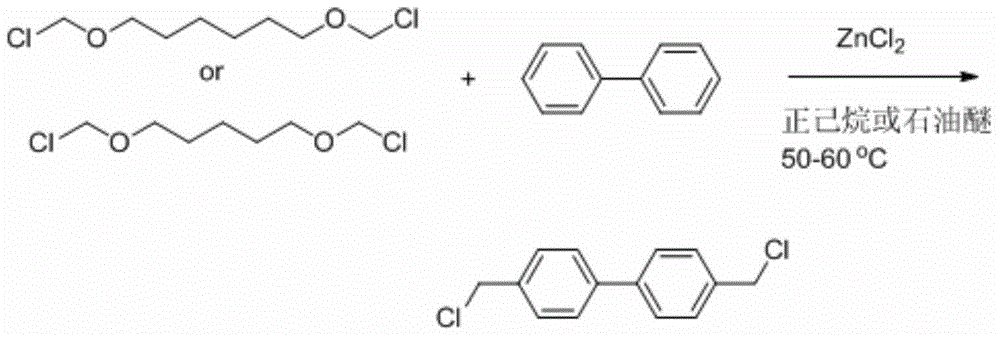
Preparation method of 4, 4'-bis(chloromethyl)-1, 1'-biphenyl
A biphenyl dichlorobenzyl and biphenyl technology, which is applied in the field of preparation of biphenyl dichlorobenzyl, can solve problems such as hidden dangers of human health and safety, and achieve the effects of low volatility, mild reaction conditions and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
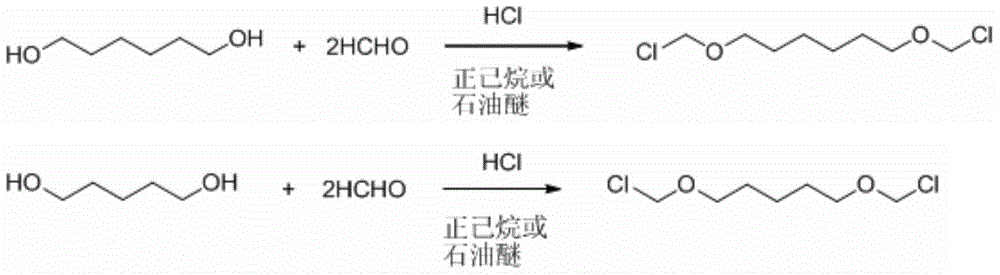
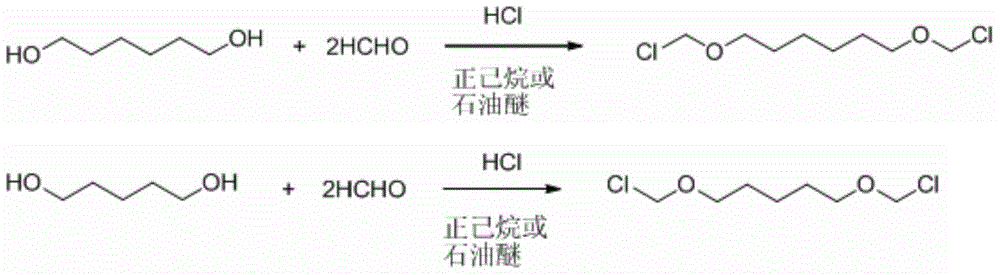
[0023] The preparation process of 4,4'-bischloromethoxy-1,1'-biphenyl of the present invention is carried out in 2 steps:
[0024] I. Add 78.701g 1,6-hexanediol (0.666mol), 40.000g paraformaldehyde (1.332mol), and 250ml n-hexane into a 500ml four-neck round bottom flask. The flask is placed in a constant temperature water bath, and the thermometer is inserted into the side port of the operator. The mercury bulb of the thermometer is located below the liquid surface; the right port is equipped with a reflux condenser, and the top of the pipe is connected to a tube. The escaping hydrogen chloride gas is absorbed by the sodium hydroxide solution. Hydrogen chloride gas is produced by the reaction of concentrated sulfuric acid and ammonium chloride and is introduced from the left port. Ammonium chloride produces more uniform bubbles than sodium chloride.
[0025] Turn on mechanical stirring. Adjust the dropping rate of concentrated sulfuric acid in the constant pressure funnel, so tha...
Embodiment 2
[0028] ⅠAdd 591.56g 1,6-hexanediol (5.006mol), 300.90g paraformaldehyde (10.02mol), 1.8L petroleum ether (re-distilled, take 60-90℃ fraction) into a 5L 4-neck flask equipped with mechanical stirring . The flask is placed in a constant temperature water bath, and the thermometer is inserted into the side port of the operator. The mercury bulb of the thermometer is located below the liquid surface; the right port is equipped with a reflux condenser, and the top of the pipe is connected to a tube. The excess hydrogen chloride gas is absorbed by the sodium hydroxide solution. Hydrogen chloride gas is produced by the reaction of concentrated sulfuric acid and ammonium chloride, and is introduced from the side port.
[0029] Turn on mechanical stirring. Adjust the dropping rate of concentrated sulfuric acid in the constant pressure funnel, so that the hydrogen chloride flow rate is controlled at 14-20L / h. Adjust the temperature of the water bath, and control the system temperature be...
Embodiment 3
[0032] I. Add 69.36g 1,5-pentanediol (0.666mol), 40.000g paraformaldehyde (1.332mol), and 300ml n-hexane into a 500ml four-necked round bottom flask. The flask is placed in a constant temperature water bath, and the thermometer is inserted into the side of the operator. The mercury bulb of the thermometer is below the liquid surface; a reflux condenser is added to the mouth, and a tube is connected to the top of the tube. The excess hydrogen chloride gas is absorbed by the sodium hydroxide solution. Hydrogen chloride gas is produced by the reaction of concentrated sulfuric acid and ammonium chloride and is introduced from the left port.
[0033] Turn on mechanical stirring. Adjust the dropping rate of the concentrated sulfuric acid in the constant pressure funnel so that the bubble generation speed in the gas scrubber is roughly 2-3 bubbles in 1 second. The temperature of the system is controlled between 15-17°C, and the hydrogen chloride gas is stopped after 2 hours of reaction....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com