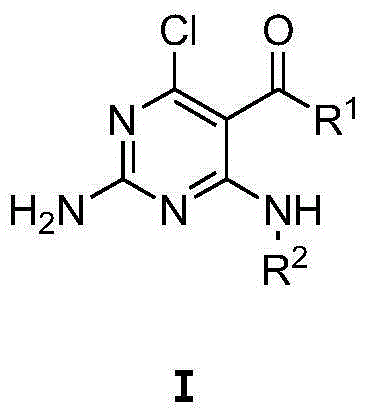
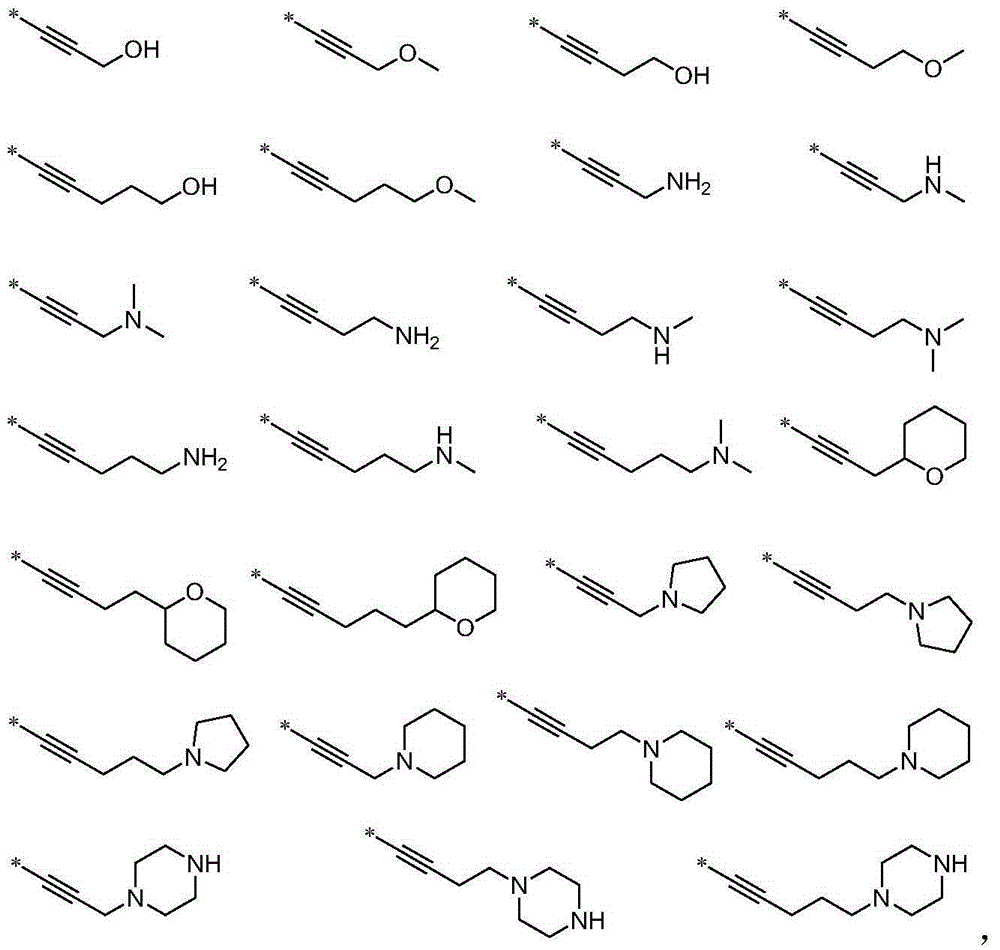
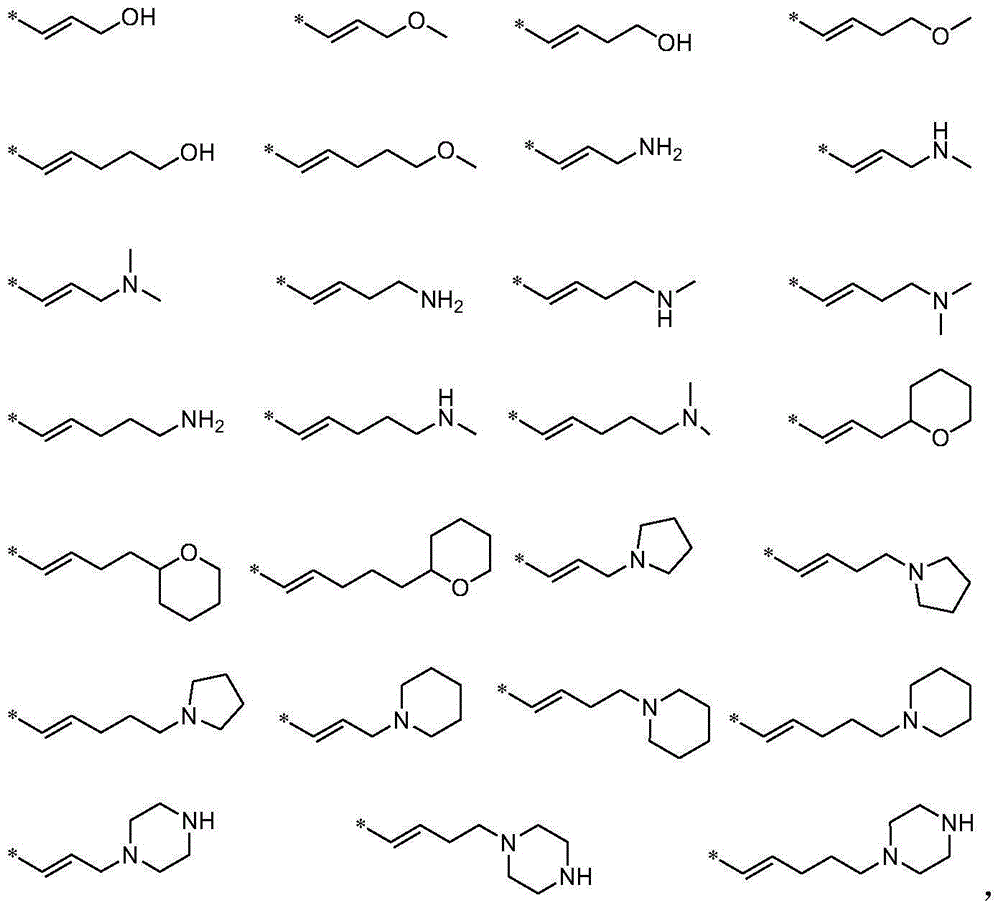
Aminopyrimidine derivative used as PPAR-gamma regulator
A phenyl, group technology, applied in the field of aminopyrimidine derivatives as PPAR-γ regulators, can solve problems such as application limitations, insufficient research and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: 1-(2-amino-4-(4-(3-ureidophenyl)benzylamino)-6-chloropyrimidin-5-yl)-6-(piperidin-1-yl)- Preparation of 2-hexyn-1-one
[0066]
[0067] The first step: N-(3-(4-formylphenyl)phenyl)urea (compound number 1)
[0068]
[0069] After deoxygenation by nitrogen bubbling in advance and continuous nitrogen protection, anhydrous potassium carbonate (27.6g, 0.2mol), palladium acetate (0.90g, 4.0mmol) and Molecular sieves (2.0g, previously activated by high-temperature roasting in a muffle furnace) were sequentially added with p-bromobenzaldehyde (18.5g, 0.1mol) and m-ureidophenylboronic acid (18.0g, 0.1mol, refer to Journal of the American Chemical Society; vol.81; ( 1959); p.3017) in a mixed solution of tetrahydrofuran (150 mL) and water (40 mL). Under the protection of nitrogen, the oil bath was heated to reflux, and the reaction was kept for about 6 hours. Ethyl acetate (300 mL) was added, stirred for half an hour, the insoluble matter was removed by filtra...
Embodiment 2
[0092] Example 2: 1-(2-amino-4-(4-(4-methoxyphenyl)anilino)-6-chloropyrimidin-5-yl)-5-methoxy-2-pentyne- Preparation of 1-keto
[0093]
[0094] The first step: 2-amino-4-(4-(4-methoxyphenyl)anilino)-6-chloropyrimidine-5-carbaldehyde (compound number 9)
[0095]
[0096] Under nitrogen protection, 4-(4-methoxyphenyl)aniline (11.4g, 57.3mmol, can refer to the literature RazlerTMetal.JournalofOrganicChemistry; vol.74; nb.3; (2009); p.1381-1384 was added Synthesis) in anhydrous tetrahydrofuran (120 mL) was cooled with an ice-water bath, and sodium hydrogen (60% mineral oil powder, 4.6 g, 114.6 mmol) was added carefully in batches. After the addition was complete, the stirring reaction was continued for about half an hour, and then 2-amino-4,6-dichloropyrimidine-5-carbaldehyde (10.0 g, 52.1 mmol) was added. The ice-water bath was removed, and the reaction system was allowed to react at room temperature for about 8-10 hours, monitored by TLC (petroleum ether:ethyl acetate=2...
Embodiment 3
[0104] Example 3: (E)-1-(2-amino-4-(4-(4-methoxyphenyl)anilino)-6-chloropyrimidin-5-yl)-6-methoxy-2 - Preparation of hexen-1-one
[0105]
[0106] The first step: (E)-1-(2-amino-4,6-dichloropyrimidin-5-yl)-6-methoxy-2-hexen-1-one (compound number 12)
[0107]
[0108] 1-(2-amino-4,6-dichloropyrimidin-5-yl)ethanone (10.0g, 48.5mmol, reference WO2005 / 28434, (2005), (A2) or WO2014 / 96423, (2014 ), (A1) to be synthesized) in anhydrous tetrahydrofuran (100mL) was cooled to -78°C with a liquid nitrogen ethanol bath, and lithium diisopropylamide (LDA, 2.0M n-hexane solution, 24.3mL, 48.5mmol ) solution, the control dripped in about half an hour. Keeping at -78°C and under the protection of nitrogen, add 4-methoxy-1-butyraldehyde (5.0 g, 48.5 mmol) dropwise, refer to the literature WO2009 / 7814, (2009), (A1), BorbasK, et al.Organic Letters; vol.10; nb.10; (2008); p.1931-1934 or ChauST, etal.Organic Letters; vol.13; nb.4; (2011); p.756-759 to synthesize) anhydrous tetrahydrofura...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com