Antibody coupled mesoporous silica/mifepristone nanometer preparation
A technology of mesoporous silica and mifepristone, which is applied in the direction of antibodies, medical preparations with inactive ingredients, inactive ingredients of polymer compounds, etc., to achieve inhibitory activity, prevent tumor metastasis, and prevent CTCs from adhering to blood vessels Endometrial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
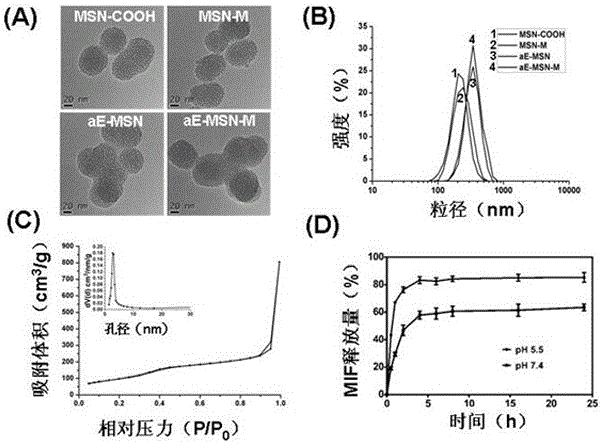
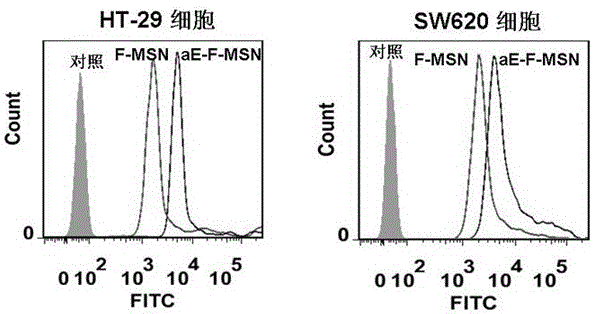
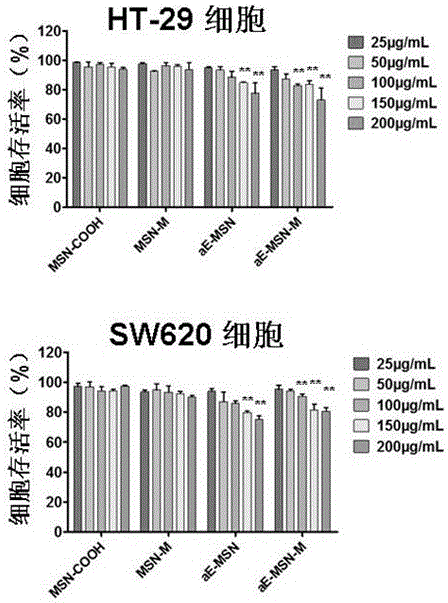
Image
Examples
Embodiment 1
[0030] (1) Measure 20mL of ultrapure water into a 50mL round bottom flask, add 2.0g of cetyltrimethylammonium chloride (CTAC), stir at room temperature until completely dissolved, add 0.02mg of triethanolamine (TEA), in 95 ℃ oil bath constant temperature heating and condensation stirring for 1h. Then 1.5 mL of tetraethylsilane (TEOS) was added dropwise, and continued to heat, condense and stir in an oil bath at 95°C for 1 h. The sample was cooled to room temperature, and washed 3-4 times by high-speed centrifugation with absolute ethanol to remove residual reaction reagents. Then collect the samples in 20mL of washing solvent (0.2g sodium chloride dissolved in 20mL methanol), stir at room temperature for 3h, and wash with high-speed methanol centrifugation. Repeat the above washing step 3-4 times to remove the templating agent CTAC. Freeze-dry to obtain white MSN solid powder.
[0031](2) Measure 2.5mL of 3-aminopropyltriethoxysilane (APTES) and 75mg of succinic anhydride i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com