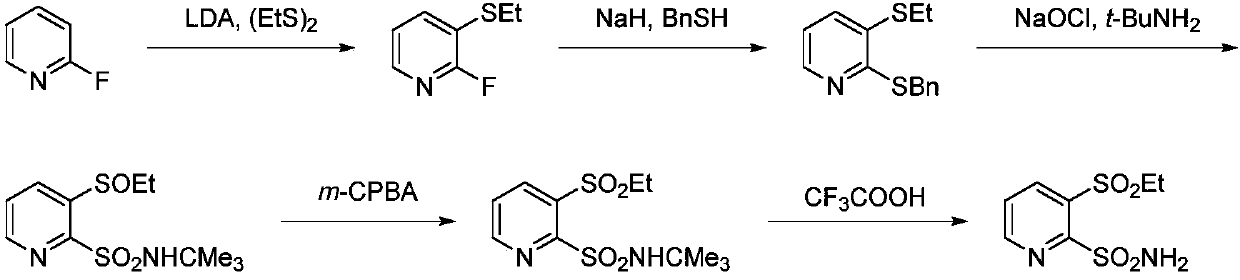
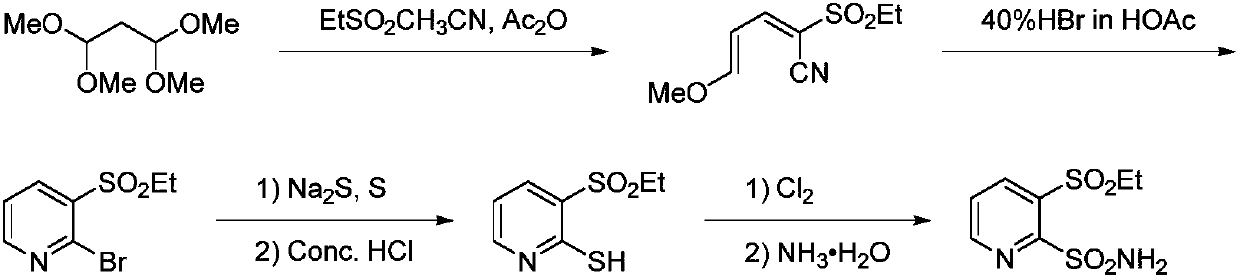
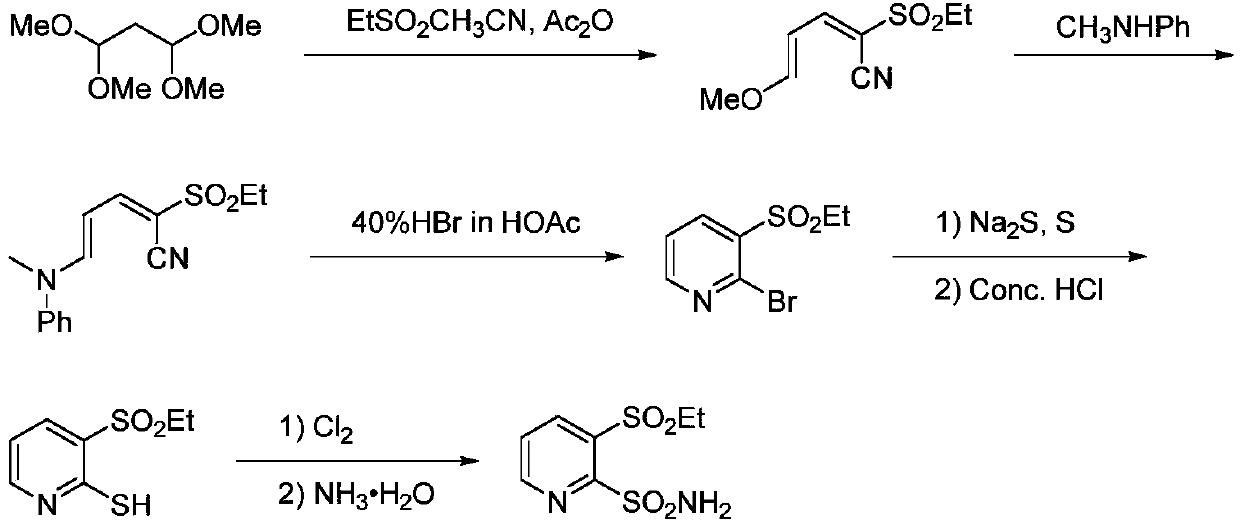
The preparation method of 3-ethanesulfonyl-2-pyridinesulfonamide and its intermediate
A technology of ethylsulfonylpyridine and ethylsulfonyl, which is applied in the field of preparation of 3-ethylsulfonyl-2-pyridinesulfonamide and its intermediates, can solve the problems of high production cost, unsuitability for industrial production, and expensive raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Mix 80.00g of sodium hydroxide (2.0mol) and 350g of N,N-dimethylformamide (DMF) in a reaction flask, cool down to 0-5°C, and slowly add ethyl sulfide dropwise under constant stirring DMF solution of alcohol (ethanethiol: 124.26g (2.0mol), DMF: 80g), keep the system temperature at 0-5°C during the dropwise addition. After dropping, continue to insulate and stir for 1 to 2 hours until the solid sodium hydroxide in the reaction bottle is completely dissolved. The system was heated up to 80-100°C, and the DMF solution of 2,3-dichloropyridine (2,3-dichloropyridine: 134.54g (0.91mol), DMF: 200g) was slowly added dropwise, and the temperature of the system was maintained at 80~100℃. After dropping, keep stirring for 3-5 hours, take a sample and control it. When the HPLC content of 2,3-dichloropyridine is less than 0.5%, the reaction is considered to be over.
[0084] After the solvent and low boilers were slowly removed under reduced pressure, 172.35 g of crude 2,3-diethylth...
Embodiment 2
[0086] Mix 112.22g of potassium hydroxide (2.0mol) and 350g of N,N-dimethylacetamide (DMA) in a reaction flask, then cool down to 0-5°C, and slowly add ethyl sulfide dropwise under constant stirring Alcohol DMA solution (ethanethiol: 124.26g (2.0mol), DMA: 80g), keep the system temperature at 0-5°C during the dropwise addition. After dropping, continue to insulate and stir for 1 to 2 hours until the potassium hydroxide solid in the reaction flask is completely dissolved to obtain a DMA solution of potassium ethanethiolate.
[0087] After dissolving 134.54g of 2,3-dichloropyridine (0.91mol) in 200g of DMA, the temperature of the system was raised to 80-100°C, and the DMA solution of potassium ethanethiolate prepared above was slowly added dropwise. Maintain the system temperature at 80-100°C. After dropping, keep stirring for 3-5 hours, take a sample and control it. When the HPLC content of 2,3-dichloropyridine is less than 0.5%, the reaction is considered to be over.
[0088...
Embodiment 3
[0090] After the 2,3-diethylthiopyridine (0.20mol) that 40.0g embodiment 1 prepares is dissolved in the glacial acetic acid of 80g, the mass concentration that slowly adds 100g is the hydrogen peroxide solution of 30% (the described mass concentration is Refers to the mass of hydrogen peroxide as a percentage of the total mass of hydrogen peroxide), with the slow addition of hydrogen peroxide, the system temperature gradually rises, when the system temperature rises to 75-80 ° C, about 1 / 3 of the hydrogen peroxide solution has been added dropwise. Stop the dropwise addition, and use an ice bath to cool down the system. When the system temperature drops to 70°C, continue to drop the remaining hydrogen peroxide solution, and maintain the system temperature at 70-85°C during the dropwise addition. After dripping, keep warm at 70-85°C for 4 hours, and take samples for control. At this time, the HPLC content of 2,3-diethylthiopyridine is 7%, and continue to keep warm at 70-85°C for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com