A kind of cephalosporin compound and preparation method thereof
A technology for cephalosporins and compounds, applied in the field of medicine, can solve the problems of low yield, easy residue, poor fluidity and the like of the preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
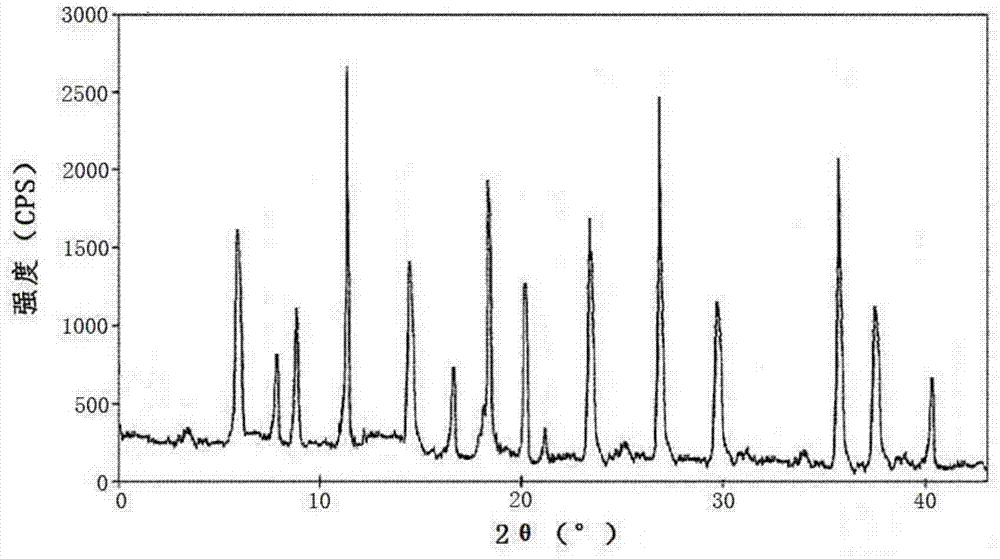
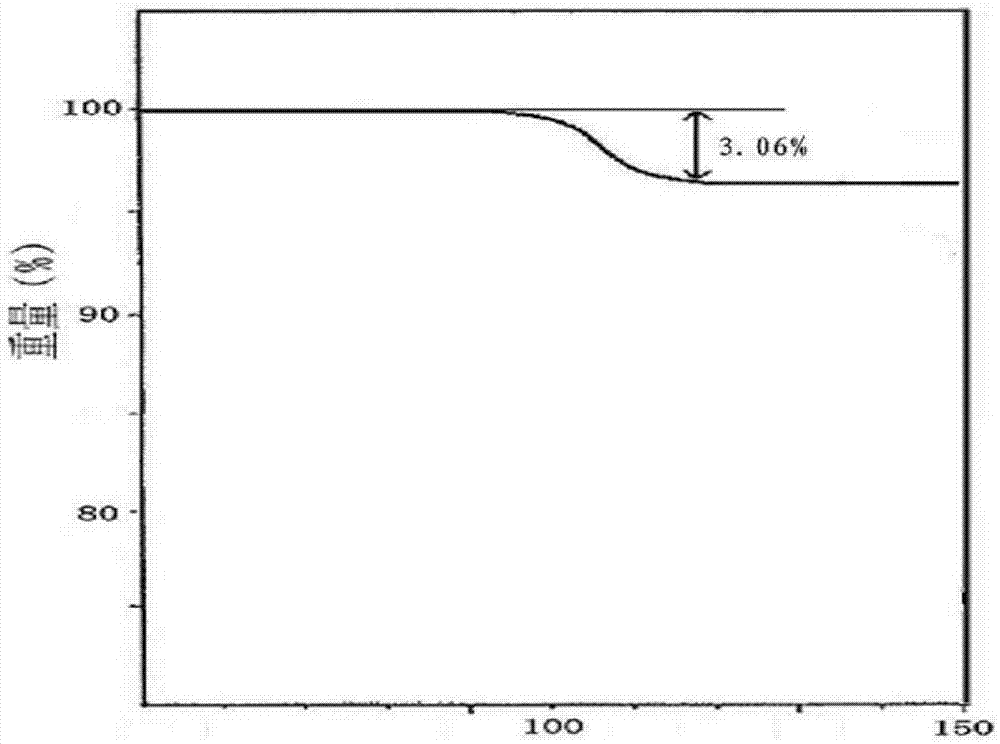
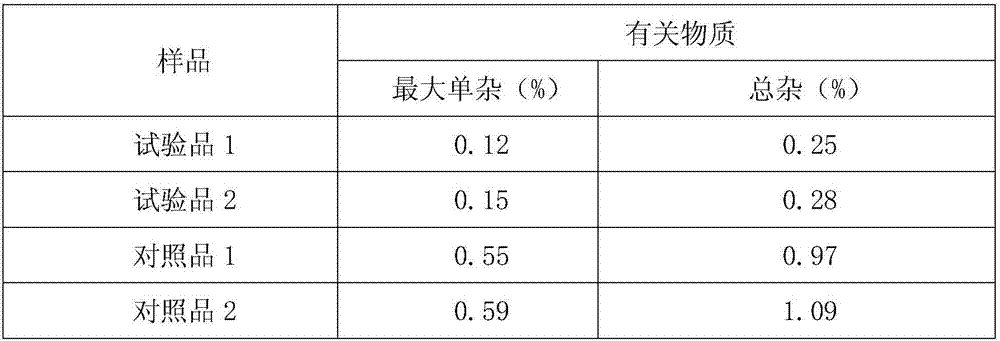
[0033] Embodiment 1: Preparation of cefazedone sodium crystal form compound
[0034] 1) Take 1 kg of crude cefzidone sodium, add it into 15 L of mixed solution A at 30-35° C., stir at a speed of 65 r / min to dissolve all of it, and obtain cefzidone sodium solution; wherein the mixed solution A Prepared for isopropanol and water at a volume ratio of 1:6;
[0035] 2) Naturally cool the cefzidone sodium solution obtained in step 1) to 15-20°C, then add the mixed solvent B at a speed of 15L / min under stirring at a stirring speed of 25r / min, after the addition is completed, cool down to 10°C , maintain the stirring speed, and grow the crystal for 3 hours;
[0036] The mixed solution B is prepared from acetone and water at a volume ratio of 1:3; the volume ratio of the mixed solution B to cefazedone sodium solution is 20:1.
[0037] 3) Cool down to 5°C, continue stirring at a stirring speed for 1 hour; keep the temperature at 0-5°C, let stand for 2 hours to crystallize, filter, was...
Embodiment 2
[0042] Embodiment 2: Preparation of cefazedone sodium crystal form compound
[0043] 1) Take 1 kg of crude cefzidone sodium, add it to 10 L of mixed solution A at 30-35° C., stir at a speed of 55 r / min to dissolve all of it, and obtain cefzidone sodium solution, wherein the mixed solution A Formulated with isopropanol and water at a volume ratio of 1:4;
[0044] 2) Naturally cool down the cefzidone sodium solution obtained in step 1) to 15-20°C, then add the mixed solvent B at a speed of 10L / min under stirring at a stirring speed of 15r / min, after the addition is completed, cool down to 10°C , maintain the stirring speed, and grow the crystal for 2 hours;
[0045] The mixed solution B is prepared from acetone and water at a volume ratio of 1:5; the volume ratio of the mixed solution B to cefazedone sodium solution is 15:1.
[0046] 3) Cool down to 5°C, continue stirring at a stirring speed for 0.5 hours; keep the temperature at 0-5°C, let stand for 4 hours to crystallize, fi...
Embodiment 3
[0050] Embodiment 3: Preparation of cefazedone sodium crystal form compound
[0051] 1) Take 1 kg of crude cefzidone sodium, add it to 13 L of mixed solution A at 30-35° C., stir at a speed of 60 r / min to dissolve all of it, and obtain cefzidone sodium solution; the mixed solution A Prepared for isopropanol and water at a volume ratio of 1:5;
[0052] 2) Naturally cool the cefzidone sodium solution obtained in step 1) to 15-20°C, then add the mixed solvent B at a speed of 12L / min under stirring at a stirring speed of 23r / min, after the addition is completed, cool down to 10°C , maintain the stirring speed, and grow the crystal for 2 hours;
[0053] The mixed solution B is prepared from acetone and water at a volume ratio of 1:4; the volume ratio of the mixed solution B to cefazedone sodium solution is 18:1.
[0054] 3) Cool down to 5°C, continue stirring at a stirring speed for 1 hour; keep the temperature at 0-5°C, let stand for 2 hours to crystallize, filter, wash the filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com