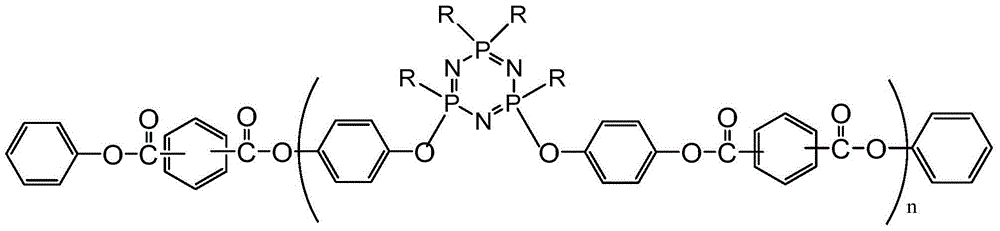
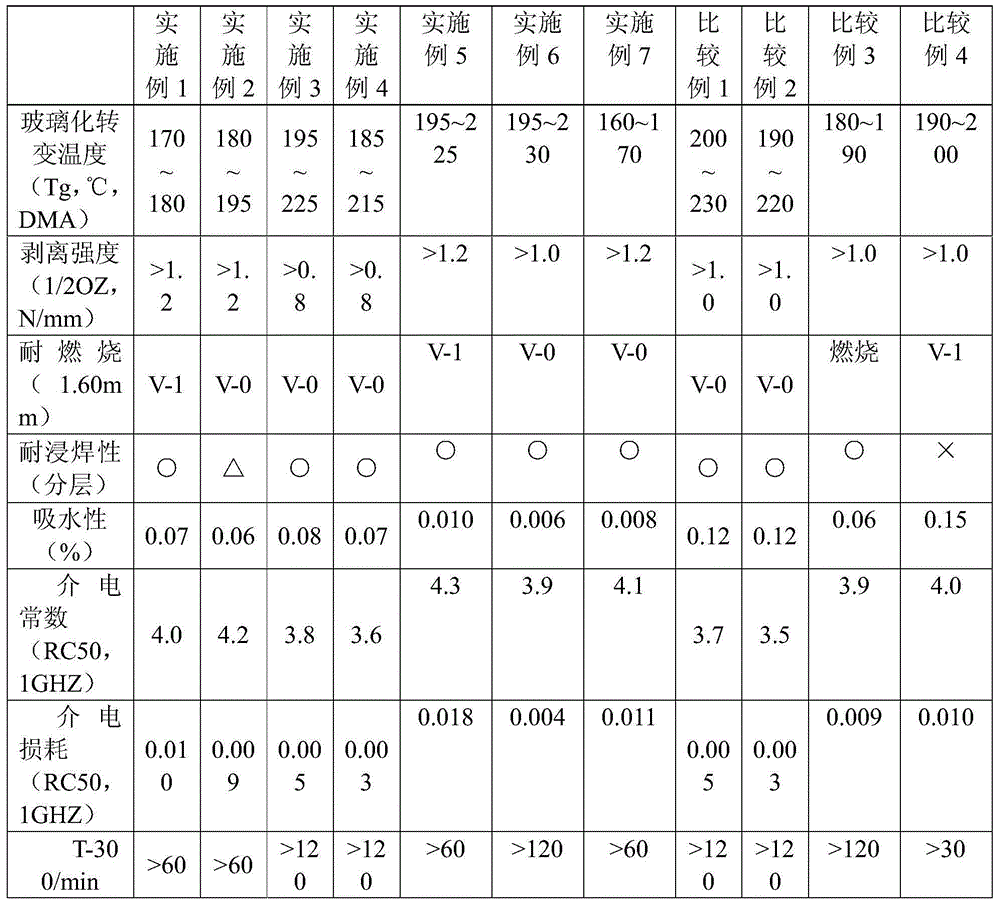
Phenoxy cyclotriphosphazene active ester, halogen free resin composition and application thereof
A technology of resin composition and phenoxy ring, which is applied in the field of phenoxycyclotriphosphazene active ester and halogen-free resin composition, can solve problems such as bad smell, influence of dielectric loss angle, pollution of environment, etc., and achieve improvement The electrical performance of the system meets the effect of halogen-free flame retardant
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add the solvent, phenoxycyclotriphosphazene containing hydroxyl groups (the proportion of which contains 2 hydroxyl groups is greater than 65%), acid-binding agent and catalyst into the reaction device, stir, pass through nitrogen protection, and gradually drop a certain amount at low temperature For p-benzoyl chloride, after reacting for 1-8 hours, add an appropriate amount of phenol, continue to react for 1-8 hours, cool to room temperature, filter with suction, distill the filtrate under pressure, and evaporate the solvent to obtain a viscous product.
[0053] After dissolving 30g of the above product in an organic solvent, add 70g of DCPD epoxy resin (the selected DCPD epoxy resin is HP-7200H (DIC), equivalent weight 275-280), an appropriate amount of imidazole and pyridine, stir and mix evenly to obtain a glue.
[0054] Select 300×300cm E-glass fiber cloth with smooth and flat surface, evenly coat the above glue, and bake it in an oven at 155°C for 7 minutes to make...
Embodiment 2
[0057] Add the solvent, phenoxycyclotriphosphazene containing hydroxyl groups (the proportion of which contains 2 hydroxyl groups is greater than 65%), acid-binding agent and catalyst into the reaction device, stir, pass through nitrogen protection, and gradually drop a certain amount at low temperature For p-benzoyl chloride, after reacting for 1-8 hours, add an appropriate amount of phenol, continue to react for 1-8 hours, cool to room temperature, filter with suction, distill the filtrate under pressure, and evaporate the solvent to obtain a viscous product.
[0058] After taking 30g of the above product and dissolving it in an organic solvent, add 40g of DCPD benzoxazine (the selected DCPD benzoxazine is LZ8260 (Huntsman)), 20g of DCPD epoxy resin (the selected DCPD epoxy resin is HP-7200H (DIC ), equivalent weight 275-280), styrene / maleic anhydride 10g (selected anhydride is EF-30, Sartomer), appropriate amount of imidazole and pyridine, stir and mix evenly to obtain glue....
Embodiment 3
[0062] Add the solvent, phenoxycyclotriphosphazene containing hydroxyl groups (the proportion of which contains 2 hydroxyl groups is greater than 65%), acid-binding agent and catalyst into the reaction device, stir, pass through nitrogen protection, and gradually drop a certain amount at low temperature For p-benzoyl chloride, after reacting for 1-8 hours, add an appropriate amount of phenol, continue to react for 1-8 hours, cool to room temperature, filter with suction, distill the filtrate under pressure, and evaporate the solvent to obtain a viscous product.
[0063] After dissolving 30g of the above product in an organic solvent, add 30g of DCPD cyanate (the selected DCPD cyanate is LONZA-PrimasetDT-4000), 20g of 4,4'-diphenylmethane bismaleimide, DCPD epoxy 20g of resin (the selected DCPD epoxy resin is HP-7200H (DIC), equivalent weight 275-280), appropriate amount of aluminum acetylacetonate and pyridine, stir and mix evenly to obtain glue.
[0064] Select 300×300cm E-gl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com