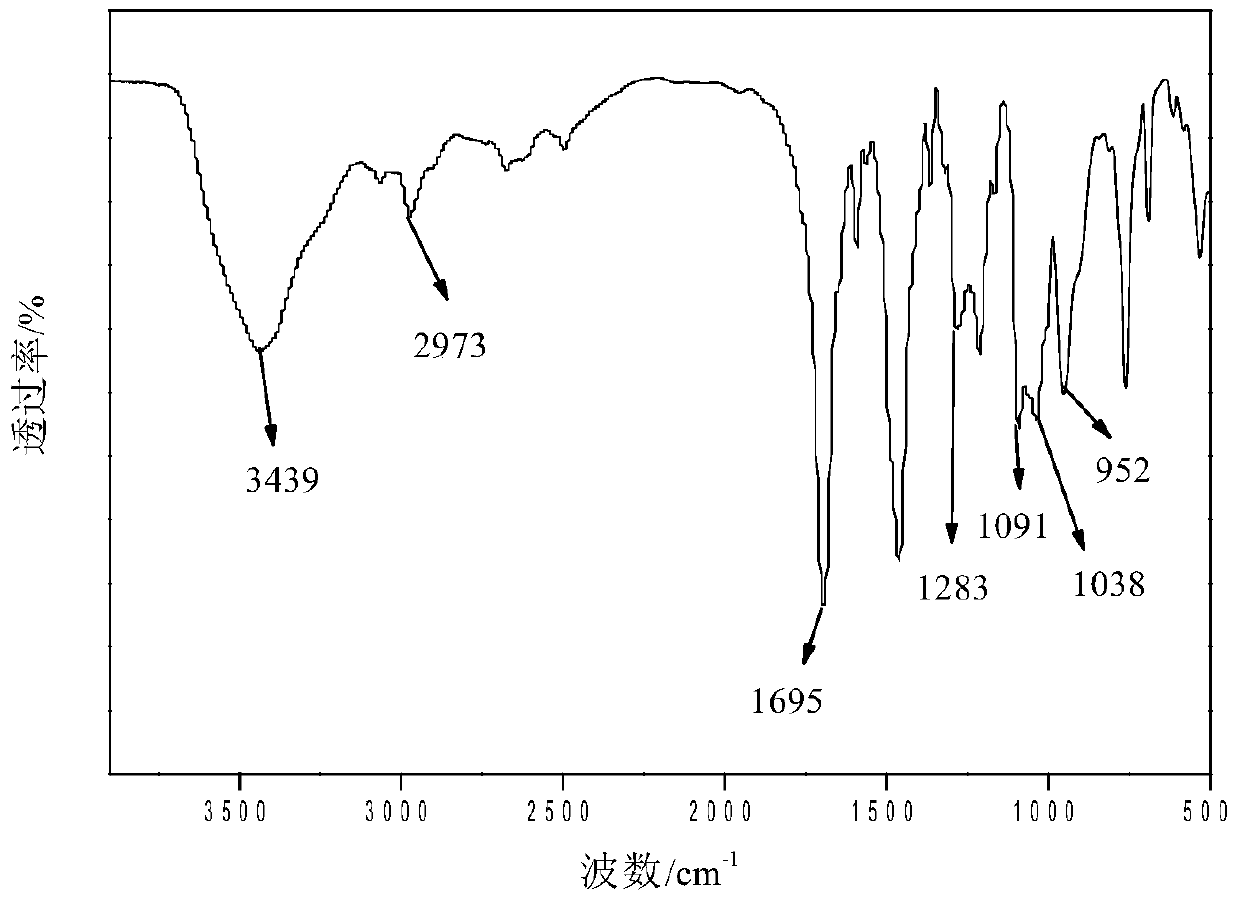
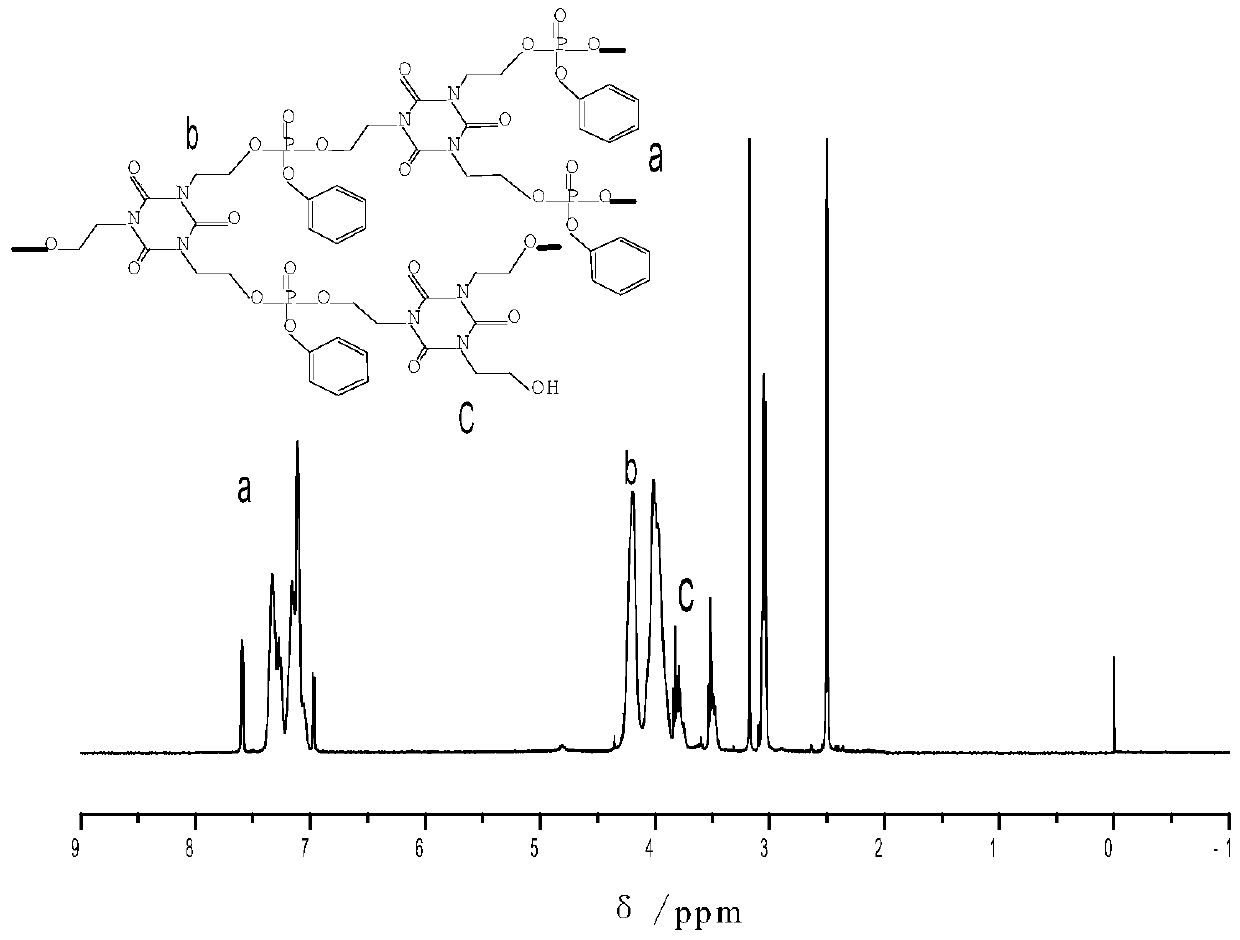
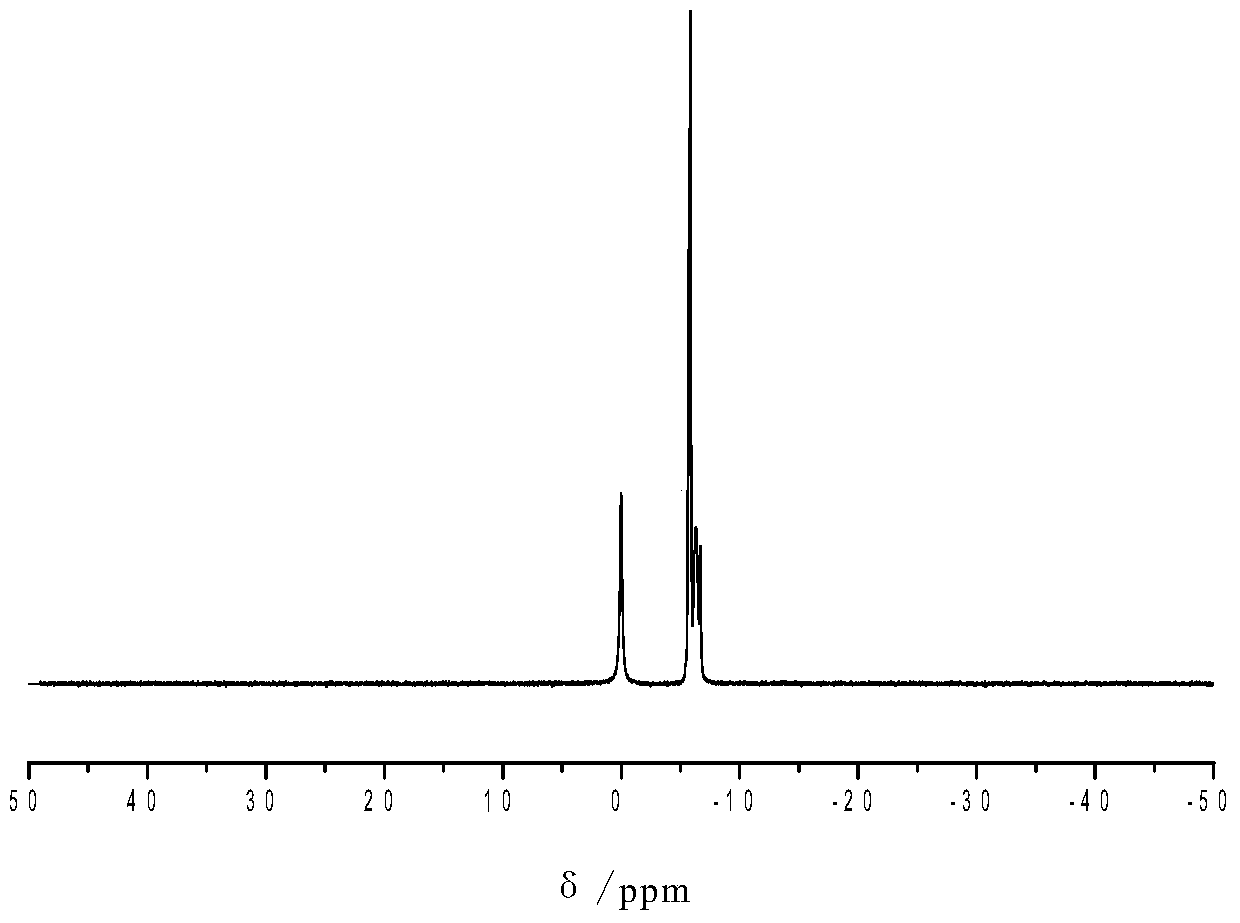
A kind of branched phosphorus-nitrogen flame retardant and preparation method thereof
A flame retardant and branched phosphorus technology, which is applied in the field of new branched phosphorus-nitrogen flame retardants and their preparation, can solve the problems of deterioration of mechanical properties of polymer matrix materials, poor compatibility of polymer materials, poor water resistance, etc. , achieve excellent flame retardant effect, good thermal stability, and reduce production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The branched phosphorus-nitrogen type flame retardant in this embodiment is based on three (2-hydroxyethyl) isocyanurate and phosphorus oxydichloride raw materials, and the branched phosphorus-nitrogen type flame retardant is obtained under the action of a catalyst and an acid-binding agent. flame retardant. Wherein, the phosphoryl dichloride is selected from phenoxy phosphoryl dichloride, the solvent is selected from pyridine, the catalyst is selected from 4-dimethylaminopyridine, and the acid-binding agent is selected from triethylamine.
[0035] a. Dissolve 6.3294g (0.03mol) of phosphoryl dichloride in 128.9215mL (126.5880g) of pyridine to obtain a phosphoryl dichloride solution, then add 6.2627g of triethylamine and 0.1848g of 4-dimethylaminopyridine; g (0.02mol) three (2-hydroxyethyl) isocyanurate was dissolved in 106.8296mL (104.8960g) pyridine to obtain three (2-hydroxyethyl) isocyanurate solution;
[0036] b. Add phosphorus oxydichloride solution to a three-nec...
Embodiment 2
[0042] The difference between this example and Example 1 is that the acid-binding agent is changed from triethylamine to pyridine.
[0043]a. Dissolve 6.3294g (0.03mol) of phosphoryl dichloride in 128.9215mL (126.5880g) of pyridine to obtain a phosphoryl dichloride solution, then add 0.1848g of 4-dimethylaminopyridine; dissolve 5.2448g (0.02mol) of tri (2-hydroxyethyl) isocyanurate was dissolved in 106.8296mL (104.8960g) pyridine to obtain three (2-hydroxyethyl)isocyanurate solution;
[0044] b. Add phosphorus oxychloride solution to the three-necked flask equipped with a stirrer, constant pressure dropping funnel and reflux condenser at -10-0°C, and add tris(2- Hydroxyethyl) isocyanurate solution, the time of dropping is 1.5h, and the insulation reaction is 0.5 hours after the dropping;
[0045] c. Raise the temperature to 40°C, keep it warm for 12 hours, and place it in an ice-water bath at 0°C to end the reaction;
[0046] d. Suction filtration, washing, and drying of the...
Embodiment 3
[0049] The difference between this example and Example 1 is that the molar mass ratio of tris(2-hydroxyethyl)isocyanurate and phosphoryl dichloride is changed from the original 1:1.5 to 1:1.
[0050] a. Dissolve 4.2196g (0.02mol) of phosphoryl dichloride in 85.9477mL (84.3920g) of pyridine to obtain a phosphoryl dichloride solution, then add 4.1751g of triethylamine and 0.1848g of 4-dimethylaminopyridine; Dissolve g (0.02mol) tris (2-hydroxyethyl) isocyanurate in 106.8296mL (104.8960g) pyridine to obtain tris (2-hydroxyethyl) isocyanurate solution, then add;
[0051] b. Add phosphorus oxydichloride solution to a three-necked flask equipped with a stirrer, a constant pressure dropping funnel and a reflux condenser at 0°C, and add tris(2-hydroxyethyl) dropwise to the three-necked flask by starvation drop method ) isocyanurate solution, dropwise for 1h, and keep warm for 1 hour after the dropwise addition;
[0052] c. Raise the temperature to 40°C, keep it warm for 12 hours, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| limiting oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com