Pharmaceutical composition for treating leuconychia
A composition and a technology for onychomycosis are applied in the field of a pharmaceutical composition for treating onychomycosis and a vesicle solution for treating onychomycosis, and can solve the problems of large dosage, insignificant effect of external coating, long medication time and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
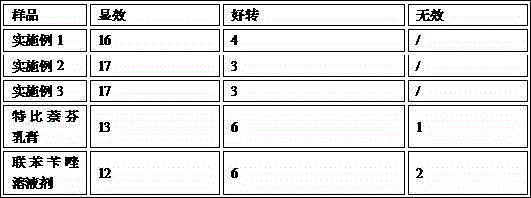
Embodiment 1
[0020] Terbinafine 1g
[0021] Bifonazole 1g
[0022] Span 4025g
[0023] Tween 8015g
[0024] Cholesterol 8g
[0025] Sodium cholate 12g
[0026] Add purified water to 100g
[0027] Preparation:
[0028] 1) Dissolve the prescription amount of terbinafine, bifonazole and capsule material in chloroform;
[0029] 2) Short-term ultrasonication forms a stable emulsion, and then removes the organic solvent by rotary evaporation under reduced pressure. After reaching a colloidal state, add the prescribed amount of hydration medium at 45°C for 1 hour of hydration, and then ultrasonically treat in an ice bath.
Embodiment 2
[0031] Terbinafine 1g
[0032] Bifonazole 1g
[0033] Span 4020g
[0034] Tween 8025g
[0035] Cholesterol 10g
[0036] Sodium cholate 10g
[0037] Add purified water to 100g
[0038] Preparation:
[0039] 1) Dissolve the prescription amount of terbinafine, bifonazole and capsule material in chloroform;
[0040] 2) Short-term ultrasonication forms a stable emulsion, and then removes the organic solvent by rotary evaporation under reduced pressure. After reaching the colloidal state, add the prescribed amount of hydration medium and hydrate at 45°C for 1 hour, and then ultrasonically treat it in an ice bath.
Embodiment 3
[0042] Terbinafine 2g
[0043] Bifonazole 1g
[0044] Span 4020g
[0045] Tween 8025g
[0046] Cholesterol 8g
[0047] Sodium cholate 10g
[0048] Add purified water to 100g
[0049] Preparation:
[0050] 1) Dissolve the prescription amount of terbinafine, bifonazole and capsule material in chloroform;
[0051] 2) Short-term ultrasonication forms a stable emulsion, and then removes the organic solvent by rotary evaporation under reduced pressure. After reaching the colloidal state, add the prescribed amount of hydration medium and hydrate at 45°C for 1 hour, and then ultrasonically treat it in an ice bath.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com