A kind of preparation method of pesticide intermediate 2,3-dichloro-5-picoline
A technology of picoline and dihydropyridine, which is applied in the field of catalytic synthesis of 2,3-dichloro-5-picoline, can solve the problems of less than 20% yield, low utilization rate of raw materials, high production cost, etc. To achieve the effect of convenient rectification and purification, low production cost and reliable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 50.20g of pyridone and 150.00g of chlorobenzene to a 500mL four-neck flask, control the temperature at about 20°C, then feed chlorine gas at 20L / hr, and react for 0.5 hours to obtain 232.60g of pyridone and chlorine addition solution.
Embodiment 2
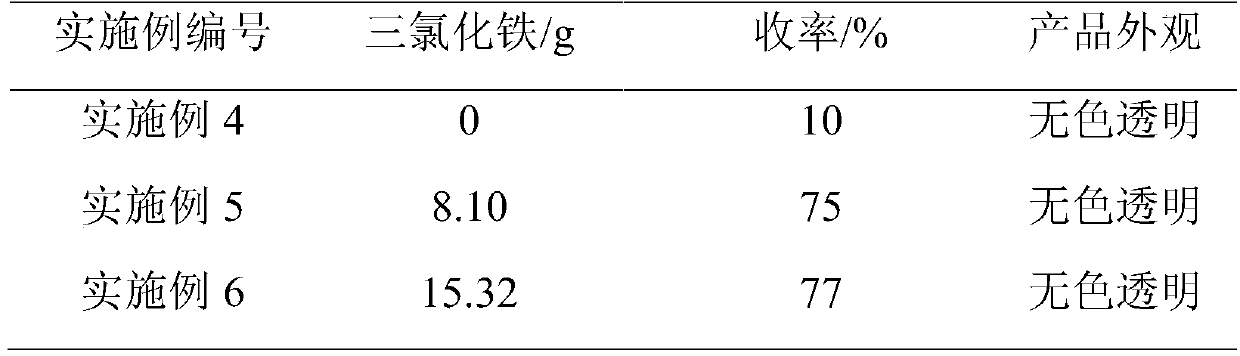
[0026] With the pyridone chloride addition solution obtained in Example 1 as raw material, add 8.10g ferric chloride and 20.23gN, N, N', N, 'N "-pentamethyldiethylenetriamine (PMDETA) body as a catalyst, control the temperature at 20°C, and continue to feed chlorine gas at 20L / hr for 0.5h.
Embodiment 3
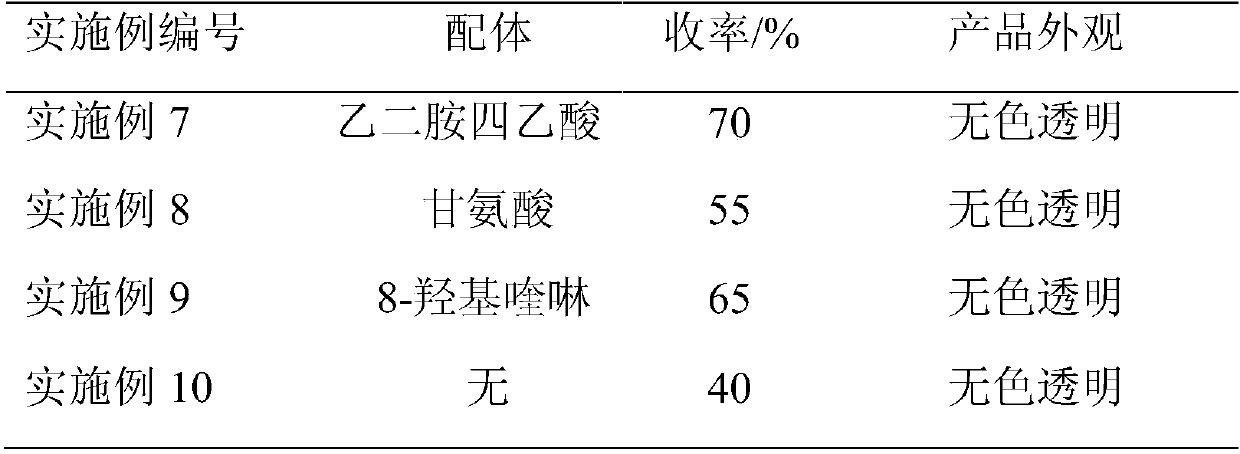
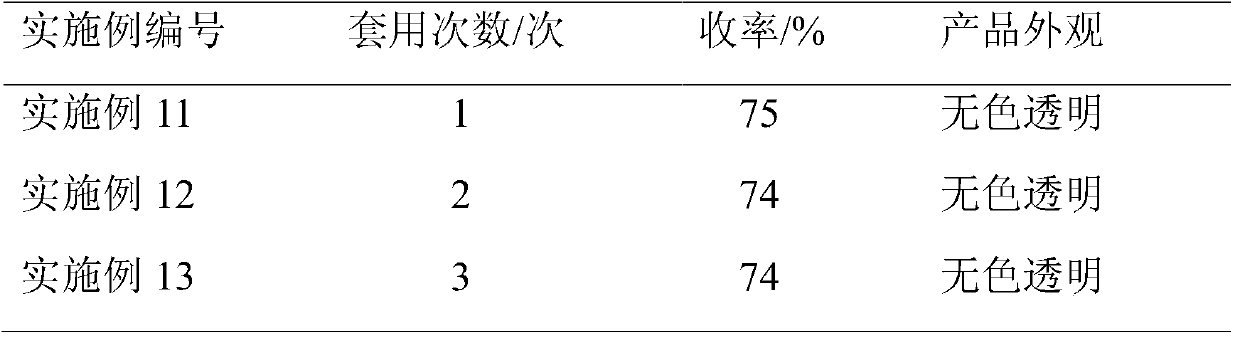
[0028] In a 500ml four-necked flask, pre-install 100.52g of chlorinated benzene solvent, heat up to reflux, then mix the reaction solution of Example 2 with phosphorus oxychloride, and then add it dropwise to the four-necked flask. After the dropwise addition, 120 The reaction was carried out at ℃ for 4 hours to obtain a dark brown reaction solution. Phosphorus oxychloride and chlorinated benzene were recovered by atmospheric distillation, and then rectified under reduced pressure to obtain 54.67 g of 2,3-dichloro-5-methylpyridine.
[0029] The gas spectrum normalized content is 99.50%, the appearance is a colorless transparent solid, and the yield of 2,3-dichloro-5-methylpyridine-pyridone is 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com