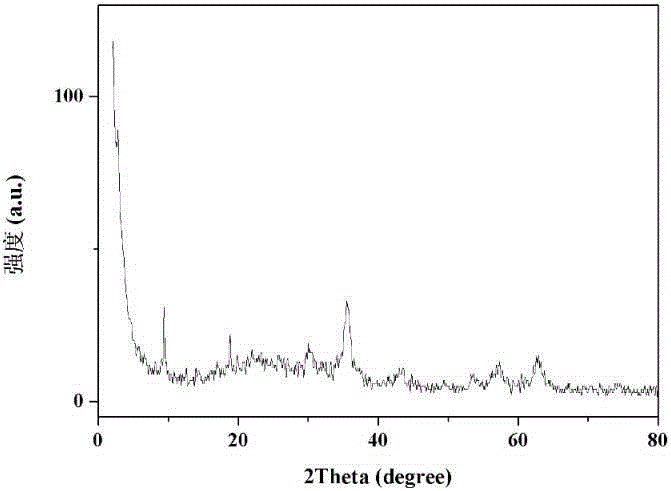
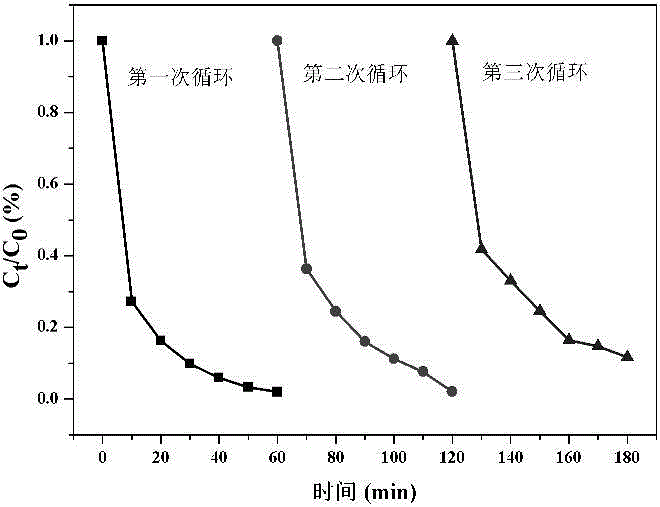
Preparing method and application of core-shell structure Fe3O4@MIL(Fe) composite material
A composite material, core-shell structure technology, applied in nanotechnology for materials and surface science, chemical instruments and methods, alkali metal compounds, etc., can solve unsatisfactory catalytic effect, incomplete degradation of organic matter, utilization of oxidants problems such as low rate, to achieve the effect of easy promotion, easy acquisition and easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Preparation of MIL-101(Fe): ultrasonically disperse 0.206g of terephthalic acid in 15mL of DMF, then add 0.675g of FeCl 3 ·6H 2 O, magnetically stirred for 15 min, reacted in a polytetrafluoroethylene-lined autoclave at 110°C for 24h; cooled to room temperature, centrifuged, washed with water and ethanol, and finally vacuum-dried at 60°C for 24h.
[0029] (2) Core-shell structure Fe 3 o 4Preparation of MIL-101(Fe) composite material: ultrasonically disperse 100mgMIL-101(Fe) in 15mL of n-octanol, and then add 0.2mL of FeCl with a concentration of 0.12mol / L 3 Ethylene glycol solution, magnetically stirred for 6h, then packaged in a polytetrafluoroethylene-lined reactor, reacted at 200°C for 8h, cooled to room temperature, washed several times with ethanol and deionized water, and dried under vacuum at 60°C.
[0030] (3) Degradation of organic wastewater: the present invention uses the organic dye Acid Orange 7 as a probe molecule to evaluate the performance of the ...
Embodiment 2
[0033] (1) Preparation of MIL-100(Fe): ultrasonically disperse 0.4102g trimesic acid in 15mLH 2 O, then add 0.605gFeCl 3 ·6H 2 O, magnetically stirred for 15 min, reacted in a polytetrafluoroethylene-lined autoclave at 150°C for 15h; cooled to room temperature, centrifuged, washed with water and ethanol, and finally vacuum-dried at 60°C for 24h.
[0034] (2) Core-shell structure Fe 3 o 4 Preparation of MIL-100(Fe) composite material: ultrasonically disperse 100mgMIL-100(Fe) in 15mL of n-octanol, and then add 0.2mL of FeCl with a concentration of 0.022mol / L 3 Ethylene glycol solution, magnetically stirred for 6h, then packaged in a polytetrafluoroethylene-lined reactor, reacted at 200°C for 8h, cooled to room temperature, washed several times with ethanol and deionized water, and dried under vacuum at 60°C.
[0035] (3) Degradation of organic wastewater: same as in Example 1.
[0036] Add 10 mg of Fe to acid orange 7 dye wastewater 3 o 4 MIL-100(Fe), Fe 3 o 4 and MIL-1...
Embodiment 3
[0038] (1) Preparation of MIL-88B(Fe): 0.348g terephthalic acid was ultrasonically dispersed in 15mLDMF and 1.2mL2mol / LNaOH mixed solution, and then 1.212gFe(NO 3 ) 3 9H 2 O, magnetically stirred for 15 min, reacted in a polytetrafluoroethylene-lined autoclave at 100°C for 12h; cooled to room temperature, centrifuged, washed with water and ethanol, and finally vacuum-dried at 60°C for 24h.
[0039] (2) Core-shell structure Fe 3 o 4 Preparation of MIL-88B(Fe) composite material: ultrasonically disperse 100mgMIL-88B(Fe) in 15mL of n-octanol, and then add 0.2mL of FeCl with a concentration of 0.12mol / L 3 Ethylene glycol solution, magnetically stirred for 6h, then packaged in a polytetrafluoroethylene-lined reactor, reacted at 200°C for 8h, cooled to room temperature, washed several times with ethanol and deionized water, and dried under vacuum at 60°C.
[0040] (3) Degradation of organic wastewater: same as in Example 1.
[0041] Add 10 mg of Fe to acid orange 7 dye wastewat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com