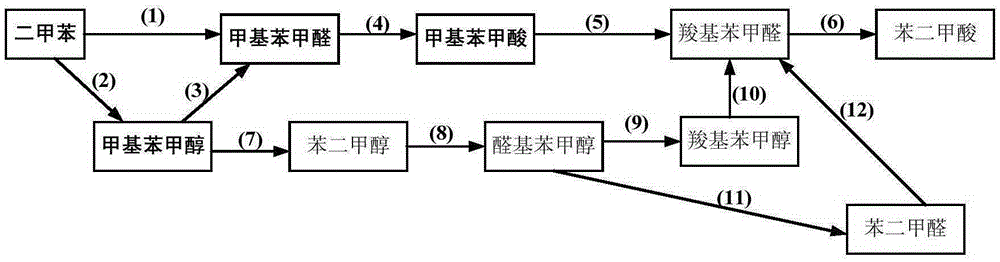
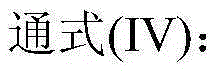Coproduction method of methyl benzyl alcohol, methyl benzaldehyde, and methyl benzoic acid
A technology of methyl benzaldehyde and methyl benzoic acid is applied in chemical instruments and methods, carboxylate preparation, carbon-based compound preparation, etc., and can solve the problems of many by-products, low product selectivity, difficult separation and the like, achieve high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
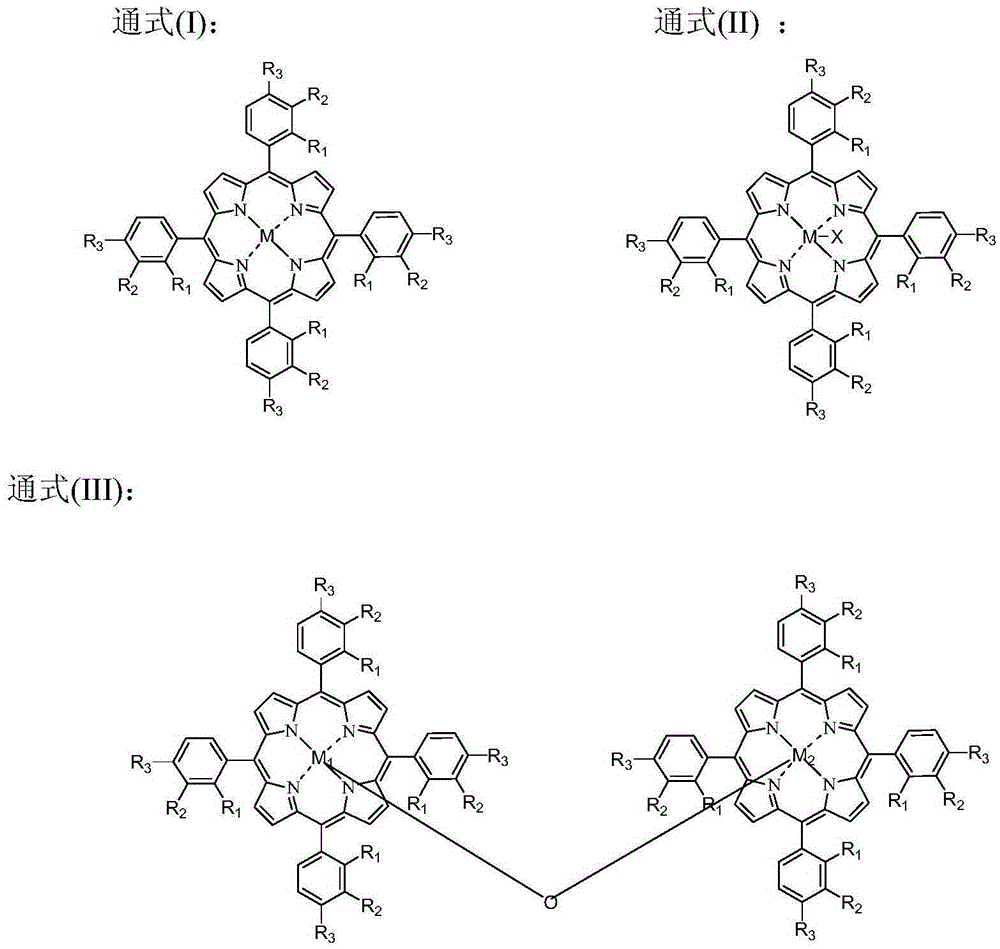
[0057] In a 1L batch oxidation reactor, add 550g of o-xylene, and add 600ppm (concentration in o-xylene) by HfO 2 , N-hydroxyl o-sulfonyl benzamide, metal phthalocyanine (R) with general formula (IV) structure 1 = H, R 2 =F, M=Fe) and metalloporphyrins (R 1 = R 3 =OH,R 2 =H, M=Ru) the mixture that forms is catalyst, and the mixture that 27.5g o-carboxybenzaldehyde and o-phthalaldehyde forms is the air that feeds 1.2MPa continuously after the co-oxidant, controls tail oxygen concentration by controlling the intake of air No more than 5%, the reactants were continuously stirred under the conditions of maintaining the pressure in the reactor at 0.8MPa and the temperature at 145°C, and the reaction was stopped after 1.6 hours, and the reactants were rapidly cooled and then sampled for analysis. The analysis results of liquid chromatography showed that the conversion rate of o-xylene was 8.8%, the total selectivity of alkyd acid in the final oxidation product was 98.5%, and the...
Embodiment 2
[0059] In a 1L batch oxidation reactor, add 550g of o-xylene, and add 660ppm (concentration in o-xylene) of N-hydroxyl-4-nitrophthalimide, having the general formula ( IV) metal phthalocyanine (R 1 =NH 2 , R 2 =H, M=Cu) and the metalloporphyrin (R 1 = R 3 = H, R 2 =CH 3 ,M 1 = M 2 =Cr) The mixture that forms is catalyzer, and 16.6g o-methylbenzaldehyde is the air that feeds 2.0MPa continuously after the co-oxidant, by controlling the intake of air, the tail oxygen concentration is controlled to be no more than 5%, and the pressure in the reactor is maintained to be The reactant was continuously stirred under the conditions of 1.6MPa and 130°C, and the reaction was stopped after 1.4 hours. The reactant was rapidly cooled and then sampled for analysis. The analysis results of liquid chromatography showed that the conversion rate of o-xylene was 6.8%, the total selectivity of alkyd acid in the final oxidation product was 98.8%, and the total selectivity of aldol was 90.9%...
Embodiment 3
[0061] In a 1L batch oxidation reactor, add 550g of p-xylene, and add 800ppm (concentration in p-xylene) of cobalt naphthenate, metal phthalocyanine (R) with general formula (IV) structure 1 =CH 3 CH 2 , R 2 =H, M=Mn), the metalloporphyrin (R 1 = R 2 = H, R 3 =CH 3 , M=Cu) the mixture that forms is catalyzer, the mixture that 99.0g p-carboxybenzyl alcohol, p-tolualdehyde and p-methyl benzyl alcohol form is the air that feeds 1.0MPa continuously after the co-oxidant, by controlling the feeding of air Quantitatively control the tail oxygen concentration to not exceed 5%, keep the reactor internal pressure at 0.5MPa, and continuously stir the reactants at a temperature of 130°C, stop the reaction after 5.0 hours, quickly cool the reactants and take samples for analysis. The analysis results of liquid chromatography showed that the conversion rate of p-xylene was 11.9%, the total selectivity of alkyd acid in the final oxidation product was 98.0%, and the total selectivity of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com