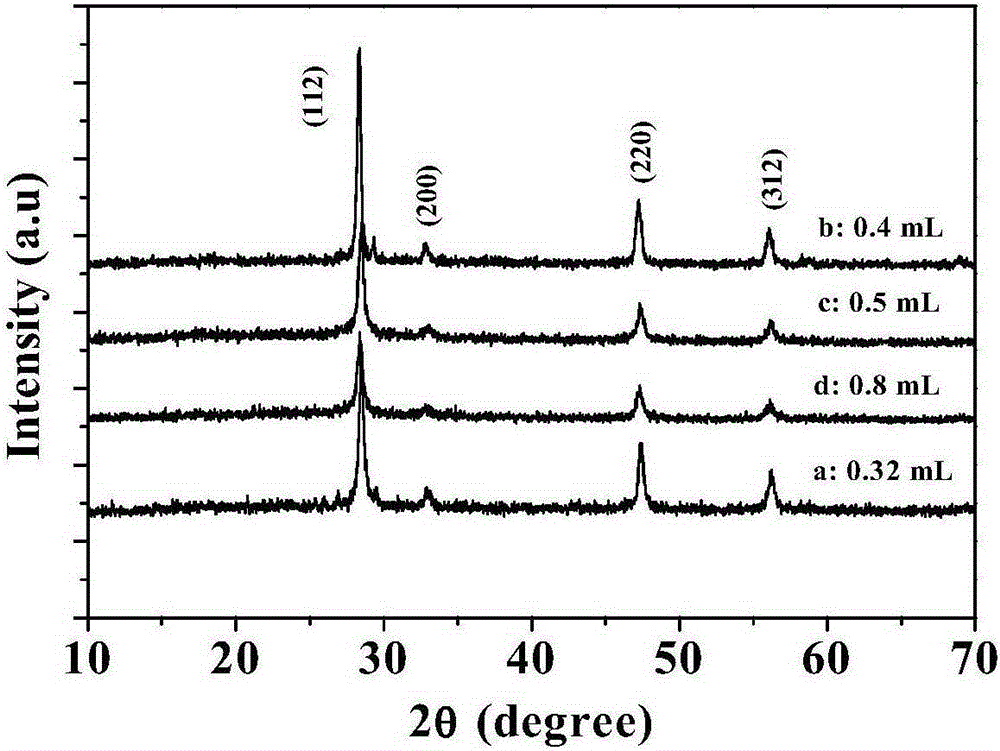
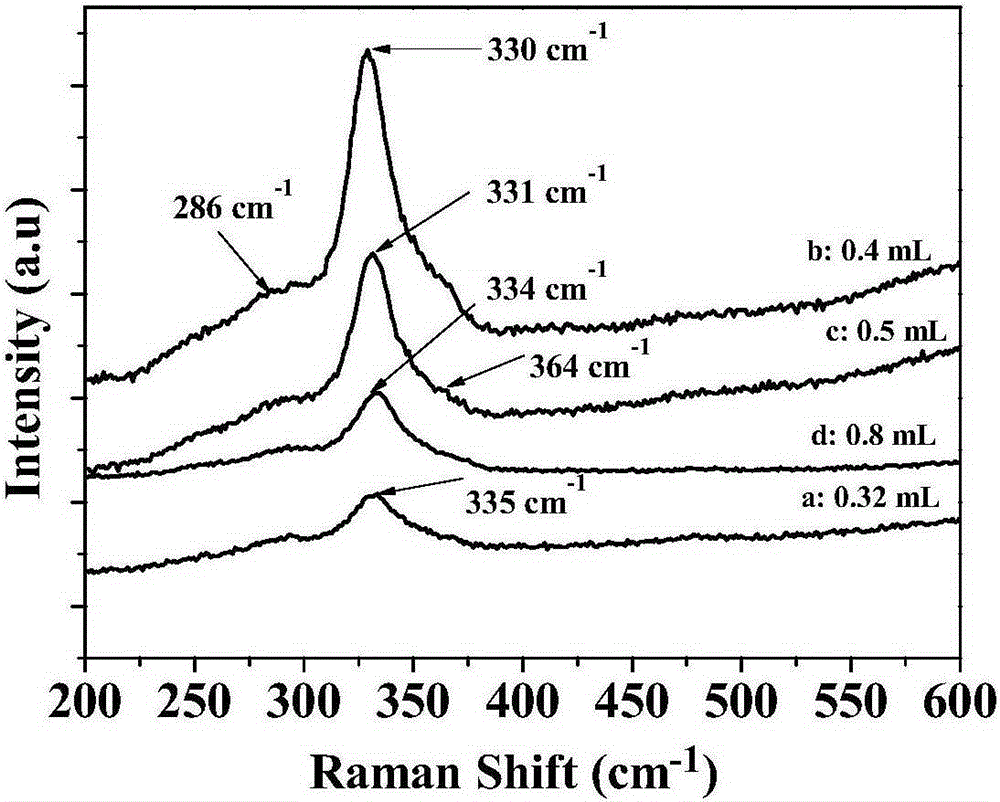
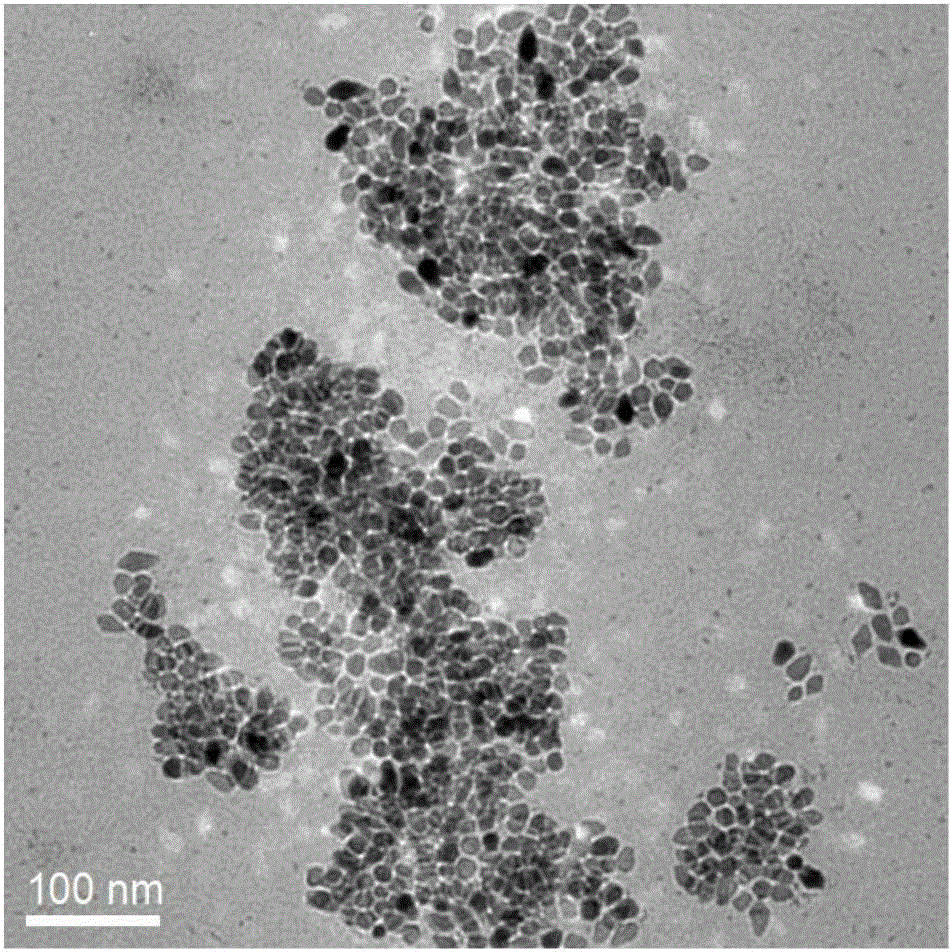
Preparation method of copper zinc tin sulfide nanocrystal with custerite structure
A copper-zinc-tin-sulfur and kesterite technology, which is applied in the fields of nanotechnology, nanotechnology, and nanotechnology for materials and surface science, can solve the problem of fast reaction speed, nanocrystal agglomeration, and difficulty in controlling nanocrystal nucleation. and growth problems, to achieve high production efficiency, reduce agglomeration, reduce the formation of binary and ternary compound chalcogenide heterophase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In this embodiment, the method for preparing copper-zinc-tin-sulfur nanocrystals with kesterite structure, the steps are as follows:
[0027] 1) Weigh 0.523g (2mmol) of copper acetylacetonate, 0.220g (1mmol) of zinc acetate and 0.225g (1mmol) of tin dichloride, put them into a three-necked flask, then add 8mL of oleylamine solvent, press 10 Raise the temperature to 100°C at a heating rate of ℃ / min, stir magnetically at a speed of 500rpm and vacuumize to remove water and oxygen for 30 minutes to obtain a stable metal-ligand complex solution;
[0028] 2) Add 0.128g (4mmol) of sulfur powder into 4mL of oleylamine solvent and ultrasonically disperse to form a transparent S-OLA solution, then add 0.32mL of DDT to prepare the S-OLA-DDT sulfur source precursor;
[0029] 3) Heat the metal-ligand complex solution prepared in step 1) to 150°C under argon, and then quickly inject the S-OLA-DDT sulfur source precursor obtained in step 2) into the metal In the ligand complex soluti...
Embodiment 2
[0033] In this embodiment, the method for preparing copper-zinc-tin-sulfur nanocrystals with kesterite structure, the steps are as follows:
[0034] 1) Weigh 0.523g (2mmol) of copper acetylacetonate, 0.220g (1mmol) of zinc acetate and 0.225g (1mmol) of tin dichloride, put them into a three-necked flask, then add 10mL of oleylamine (OLA) solvent , the temperature was raised to 120°C at a heating rate of 20°C / min, magnetically stirred at a speed of 700rpm and vacuumed to remove water and oxygen for 30min to obtain a stable metal-ligand complex solution;
[0035] 2) Add 0.128g (5mmol) of sulfur powder into 4mL of oleylamine solvent and ultrasonically disperse to form a transparent S-OLA solution, then add 0.4mL of dodecanethiol (DDT) to prepare the sulfur source precursor;
[0036] 3) Heating the metal-ligand complex solution prepared in step 1) to 160°C under argon gas, and then quickly injecting the sulfur source precursor obtained in step 2) into the metal-ligand complex solut...
Embodiment 3
[0040] In this embodiment, the method for preparing copper-zinc-tin-sulfur nanocrystals with kesterite structure, the steps are as follows:
[0041] 1) Weigh 0.523g (2mmol) of copper acetylacetonate, 0.220g (1mmol) of zinc acetate and 0.225g (1mmol) of tin dichloride, put them into a three-necked flask, then add 12mL of oleylamine (OLA) solvent , the temperature was raised to 130°C at a heating rate of 30°C / min, magnetically stirred at a speed of 800rpm and vacuumed to remove water and oxygen for 30min to obtain a stable metal-ligand complex solution;
[0042] 2) Add 0.128g (6mmol) of sulfur powder into 4mL of oleylamine solvent and ultrasonically disperse to form a transparent S-OLA solution, then add 0.5mL of dodecanethiol (DDT) to prepare the sulfur source precursor;
[0043] 3) Add the metal-ligand complex solution prepared in step 1) to 170°C under argon, and then quickly inject the sulfur source precursor obtained in step 2) into the metal-ligand complex solution prepare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com