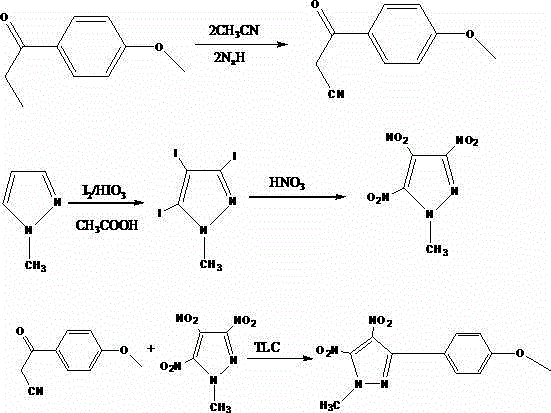
Synthesis method of 2-methyl-3,4-binitro-5-(methoxyphenyl) pyrazole
A technology of methoxyphenyl and synthesis method, which is applied in the synthesis field of 2-methyl-3,4-dinitro-5-pyrazole, can solve the problems of poor thermal stability, easy thermal decomposition and the like, and achieves thermal stability Sex-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Add 16g of methyl p-methoxybenzoate and 80mL of toluene in a 250mL round-bottomed three-necked flask, and then fully stir until methyl p-methoxybenzoate is completely dissolved. After adding 6g of 0.2mol sodium hydride, place it in a water bath Heat to 70°C, and at the same time add 10mL of 0.25mol / L acetonitrile solution dropwise to the three-necked flask, while controlling the dropping time to ensure that the dropwise addition is completed within 2 hours; after the dropwise addition is completed, heat it up to 90°C and continue stirring React for 20 hours, after the yellow substance is formed, detect the reaction by thin layer chromatography, after the completion of the detection reaction by thin layer chromatography, suction filter it, collect the yellow precipitate for the first time, and slowly stir the filtrate, add 20mL of glacial acetic acid, after cooling to 20°C, add 100mL of ice water, filter again to collect the filter cake and combine with the first yellow...
example 2
[0021] Add 16.5g of methyl p-methoxybenzoate and 81mL of toluene in a 250mL round-bottomed three-neck flask, then fully stir until methyl p-methoxybenzoate is completely dissolved, after adding 0.2mol sodium hydride of 6.5g, to Heat it in a water bath to 72°C, and at the same time add 11mL of 0.25mol / L acetonitrile solution dropwise to the three-necked flask, while controlling the dropping time to ensure that the dropwise addition is completed within 2 hours; after the dropwise addition is completed, heat it up to 90°C , continue stirring and reacting for 21h, after a yellow substance is generated, detect the reaction by thin-layer chromatography, after the completion of the detection reaction by thin-layer chromatography, suction filter it, collect the yellow precipitate for the first time, and slowly stir the filtrate 21mL of glacial acetic acid was added, and after it was cooled to 25°C, 102mL of ice water was added, and the filter cake was collected by suction filtration ...
example 3
[0023] Add 17g of methyl p-methoxybenzoate and 82mL of toluene to a 250mL round-bottomed three-necked flask, and then fully stir until methyl p-methoxybenzoate is completely dissolved. After adding 7g of 0.2mol sodium hydride, place it in a water bath Heat to 75°C, and add 12mL of 0.25mol / L acetonitrile solution dropwise to the three-necked flask at the same time, while controlling the dropping time to ensure that the dropwise addition is completed within 2 hours; after the dropwise addition is completed, heat it up to 90°C and continue Stir the reaction for 22 hours. After a yellow substance is generated, detect the reaction by thin-layer chromatography. After the detection reaction by thin-layer chromatography is completed, perform suction filtration to collect the first yellow precipitate, and slowly stir the filtrate. Add 22mL of glacial acetic acid, after cooling to 30°C, add 105mL of ice water, filter the filter cake again and combine it with the first yellow precipitate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com