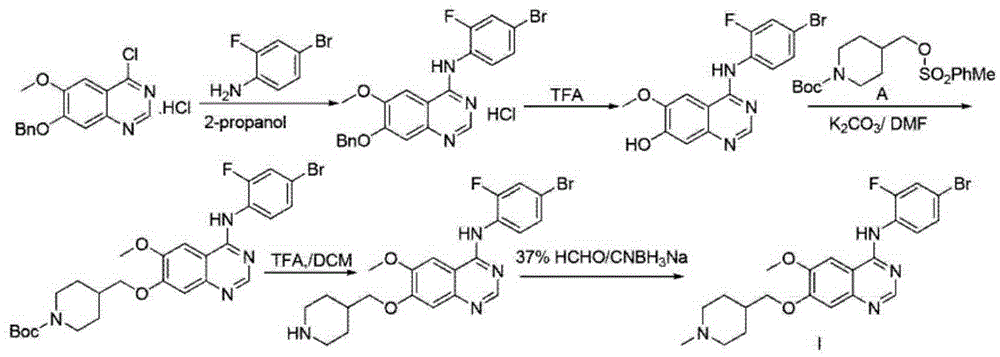
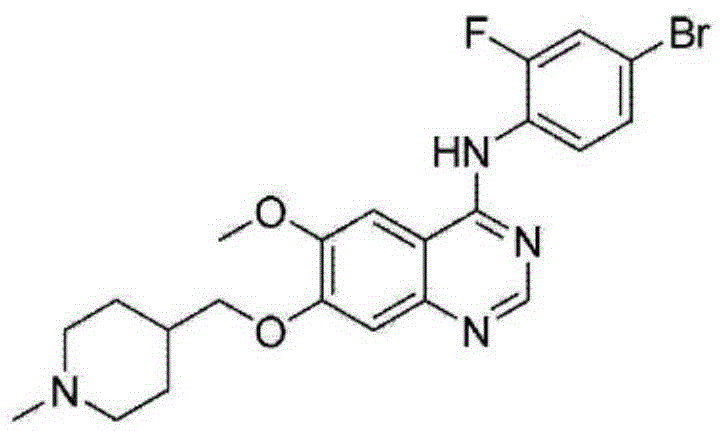
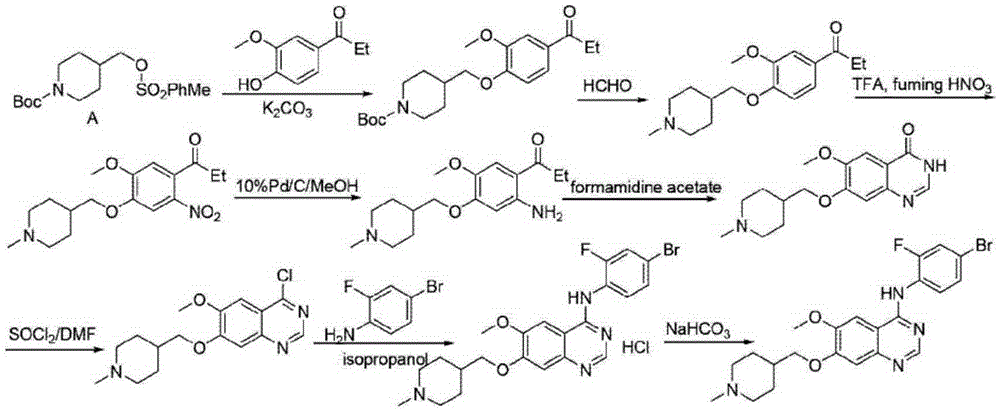
Method for synthesizing Vandetanib compound
A synthetic method, the technique of vandetanib, applied in the field of synthesis of vandetanib compounds, can solve the problems of cumbersome synthetic routes, high production costs, harmful environment, etc., and achieve the advantages of reduced process steps, short preparation cycle and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the preparation of Vandetanib
[0050]Step 1): Add 45.0g (300mmol) of compound I, 49.7g (360mmol) of potassium carbonate, and 450ml of DMF to the reaction flask, and stir at a temperature of 15°C for about 30 minutes. After the solution is dissolved, add 110.9g (300mmol) of compound II and heat up React at 80°C for 10 hours. After the reaction is completed, add the reaction liquid to 20 times of water to precipitate a solid, which is filtered and dried to obtain 98.9 g of Compound III, with a yield of 95.2% and a purity of 99.62% (HPLC);
[0051] Step 2): Compound III 90.1g (260mmol) was dissolved in 300ml of acetic acid, stirred, and 68% HNO was slowly added to the reaction flask 3 17.0ml and 98% H 2 SO 4 55.7ml of the mixture was added within 0.5 hours and reacted at 0°C for 1 hour. After the reaction was completed, it was cooled to room temperature. Add ice water to cool, adjust the pH to neutral with 20% sodium hydroxide aqueous solution, stir for 5...
Embodiment 2
[0057] Embodiment 2: the preparation of Vandetanib
[0058] Step 1): Add 45.0g (300mmol) of compound I, 49.7g (360mmol) of potassium carbonate, and 675ml of DMF to the reaction flask, and stir at a temperature of 15°C for about 30 minutes. After the solution is dissolved, add 221.7g (600mmol) of compound II and heat React at 100°C for 5 hours. After the reaction is completed, add the reaction liquid to 20 times of water, and a solid precipitates, which is filtered and dried to obtain 101.1 g of Compound III, with a yield of 97.3% and a purity of 99.62% (HPLC);
[0059] Step 2): Compound III 90.1g (260mmol) was dissolved in 300ml of acetic acid, stirred, and 68% HNO was slowly added to the reaction flask 3 34.0ml and 98% H 2 SO 4 111.4ml of the mixture was added within 1 hour and reacted at 20°C for 2 hours. After the reaction was complete, it was cooled to room temperature. Add ice water to cool, adjust the pH to neutral with 20% sodium hydroxide aqueous solution, stir for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com