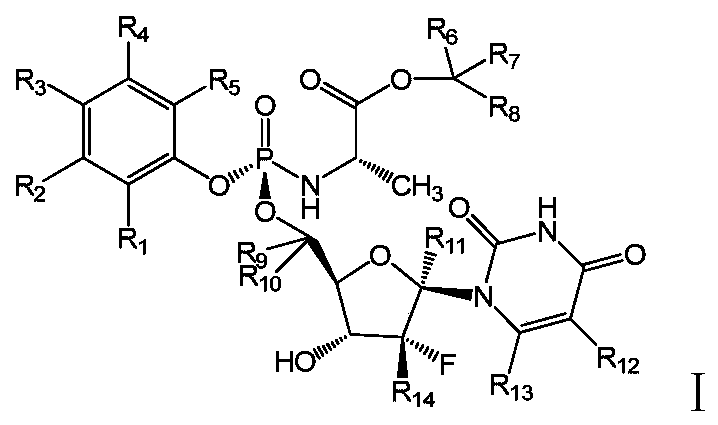
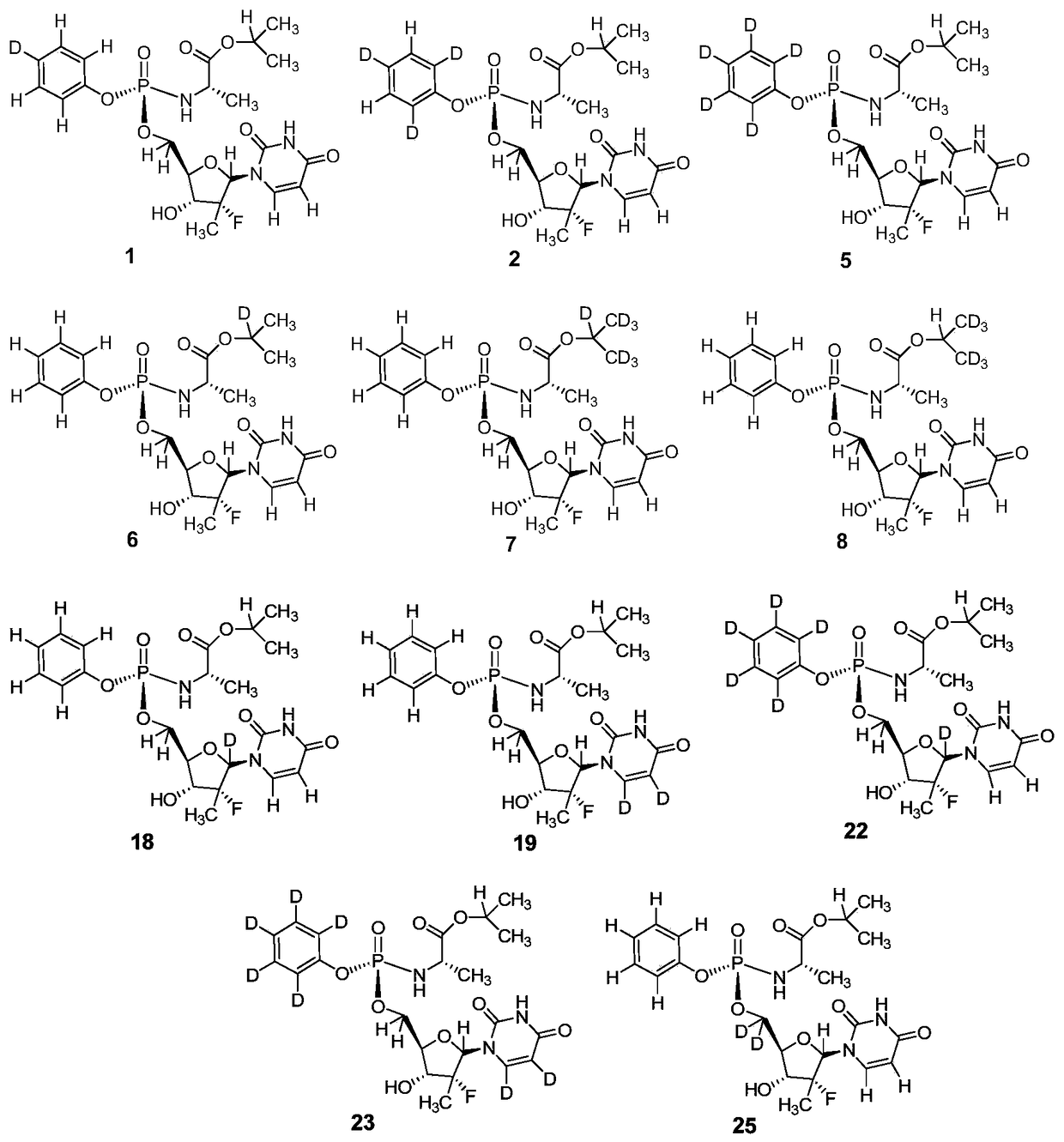
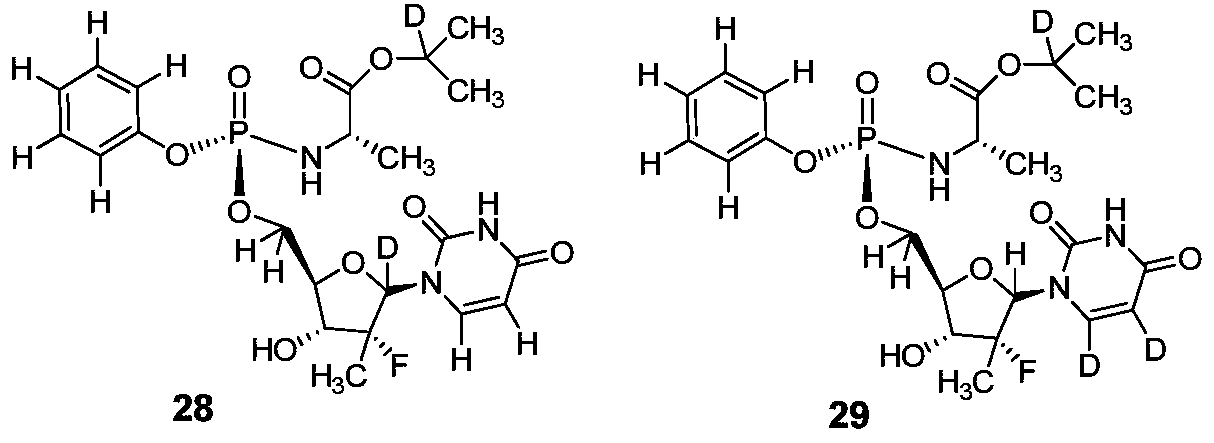
deuterated nucleoside derivatives
A technology for medicines and compounds is applied in the field of preparation of medicines for treating hepatitis C virus infection, and can solve the problems of high treatment cost and long treatment period.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of Compound 1
[0062] step 1)
[0063]
[0064] Weigh 4-bromophenol (5.03g, 29mmol) in a 100ml three-necked flask, add deuterated ethanol-d125ml (22.30g, 474.5mmol) and triethylamine (8.81g, 87mmol), stir at room temperature for 5min, then add 10% anhydrous Pd / C (0.52g), under stirring with N 2 Replace the air in the three-necked bottle for 5 times, and then use D 2 replace N 2 3 times and kept at room temperature overnight. The reaction solution was filtered to remove Pd / C, and the filtrate was rotary evaporated to obtain a yellow oily residue (1.82g). The residue was subjected to silica gel column chromatography, and the mobile phase was dichloromethane-ethyl acetate (100:1, v / v) , to obtain 4-deuterated phenol (1.44g, 15.14mmol, yield 52%, HPLC detection purity 99.37%).
[0065] 1 H-NMR (CDCl 3 , 300MHz): δ7.24-7.21 (d, 2H, o-aromatic), 6.85-6.82 (d, 2H, m-aromatic).
[0066] step (2)
[0067]
[0068] Weigh POCl in a three-necke...
Embodiment 2
[0077] Example 2 Preparation of Compound 5
[0078] step 1)
[0079]
[0080] Weigh phenol (4.00g, 42.5mmol) in a 35ml microwave reaction tube, add heavy water 18ml (19.9g), Cr powder (200mg, 3.85mmol), add 35% DCl-heavy aqueous solution (3.32g, 28.0mmol) under stirring , Stir for 1 hour, extend the nitrogen gas under the liquid surface through the needle to replace the generated deuterium gas, seal the microwave reaction tube with the microwave reaction bottle cap, and microwave the reaction. Set microwave reaction parameters temperature T = 200°C, pressure P max =250psi, power P=150w, reaction time t=2 hours. After the reaction was completed, let it cool, and the reaction solution was extracted with anhydrous ether (2×30ml), and the organic layers were combined and dried over anhydrous sodium sulfate. Filtration, concentrating under reduced pressure to remove the solvent gave a light yellow oily substance, which precipitated anhydrous needle crystals after cooling down...
Embodiment 3
[0096] Example 3 Preparation of Compound 6
[0097] step 1)
[0098]
[0099] Add 250ml ether, 8.0g LiAlD to a 500ml three-necked reaction flask 4 , Add dropwise a mixed solvent of 18.4g acetone and 50ml diethyl ether under an ice bath, and finish adding in about 2 hours. After the addition, heat to reflux for 2 hours, cool down, add 8.0ml of water, 8.0ml of 15% NaOH, and 24.0ml of water in sequence, filter, dry over anhydrous magnesium sulfate, and collect fractions at 78°C by atmospheric distillation to obtain 16.37g of liquid, namely the compound 6-a, the yield is 83.3%. MS (M+H): 62.3 (deuterium content >98.5%).
[0100] step (2)
[0101]
[0102] 7 g of compound 6-a, 1 g of L-alanine, and 6.1 g of trimethylchlorosilane were added to a microwave reaction test tube, and microwaved at 120° C. for 10 minutes. After the reaction, the solvent was concentrated to dryness to obtain an oily substance, which was beaten by adding an appropriate amount of diethyl ether, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com