Hand-foot-and-mouth disease resistant drug activity detection method and kit
A technology of drug activity and detection method, which is applied in the field of medicine to achieve the effect of good repeatability, small workload, fast and accurate experiment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1 anti-hand, foot and mouth disease drug activity screening method
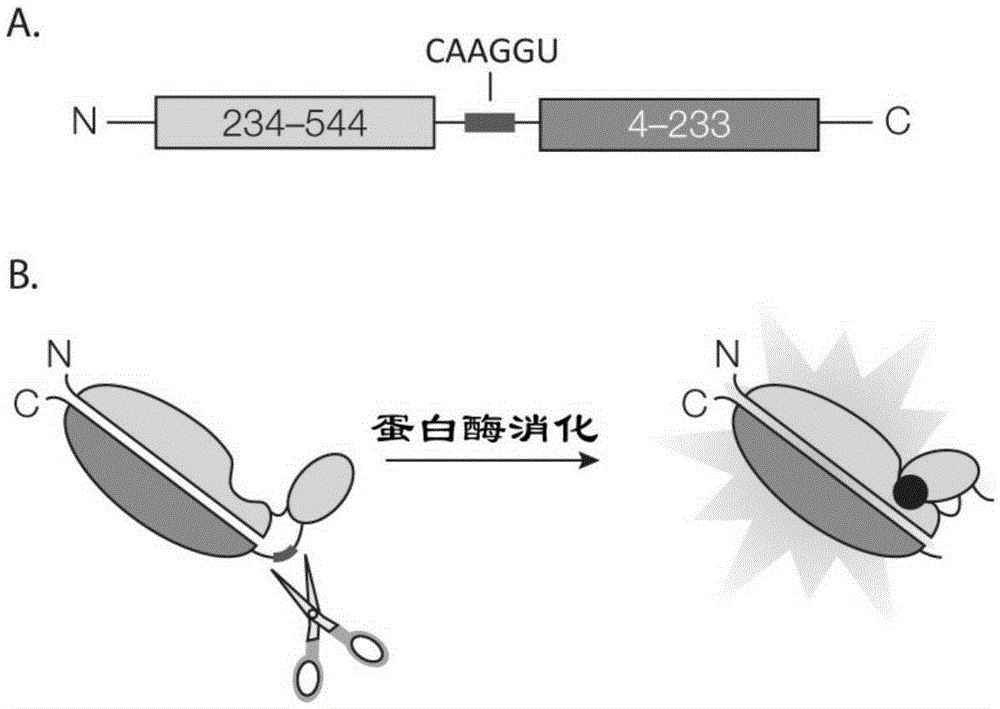
[0089] 1. Load the nucleotide sequence (CAAGGU) corresponding to the substrate Gln-Gly of 2A and 3C proteases into pGloSensor TM -10F plasmid (Promege Company), utilizes cell-free in vitro transcription and translation system to synthesize substrate recombinant luciferase;
[0090] 2. In vitro recombinant expression of 2A and 3C proteases of each hand, foot and mouth virus, including EV71, CoxA16, CoxA2, CoxA4, CoxA5, CoxA7, CoxA9, CoxA10, CoxB1-5 and 2A and 3C proteases of ECHO virus;
[0091] 3. Incubate the obtained recombinant luciferase with 2A and 3C proteases and drugs at 30°C for 1-4 hours, and detect the bioluminescence intensity (RLU) of luciferin.
[0092] The protease reaction system is:
[0093] Substrate: 1~10μg
[0094] Protease: 10~100IU
[0095] Test substance: >0μmol / L
[0096] Hepes buffer (10~100mM,
[0097] pH7.0~8.0): Make up to 100μL
[0098] Taking no test su...
Embodiment 2
[0105] Embodiment 2 trans-epoxysuccinyl-L-leucyl amido (4-guanidino) butane (E-64) inhibition test
[0106] Using the recognized cysteine protease inhibitor trans-epoxysuccinyl-L-leucyl amido (4-guanidino) butane to study its inhibitory effect on the activity of various hand, foot and mouth virus 2A and 3C proteases, verify reliability and applicability of this method.
[0107] 1. Materials and methods
[0108] 1.1 Substrate
[0109] In pGloSensor TM The CAAGGU fragment was inserted into the -10F plasmid, and a luciferase containing Gln-Gly amino acid residues was synthesized after in vitro cell-free transcription and translation, provided by Promege.
[0110] 1.2 Protease
[0111] The 2A and 3C proteases of EV71, CoxA16, CoxA2, CoxA4, CoxA5, CoxA7, CoxA9, CoxA10, CoxB1-5, and ECHO virus were expressed and purified in vitro by Promega, with a purity of >85%.
[0112] 1.3 Reagents
[0113] Trans-epoxysuccinyl-L-leucylamino (4-guanidino) butane (E-64) was purchased fro...
Embodiment 3
[0125] Embodiment 3 investigates the inhibition of different antiviral drugs to EV71 virus 3C protease
[0126] Adopt the method described in the present invention, aim at the screening verification of existing antiviral compound inhibiting EV71 virus 3C protease activity
[0127] 1. Materials and methods
[0128] 1.1 Substrate
[0129] In pGloSensor TM The CAAGGU fragment was inserted into the -10F plasmid, and a luciferase containing Gln-Gly amino acid residues was synthesized after in vitro cell-free transcription and translation, provided by Promege.
[0130] 1.2 Protease
[0131] The 3C protease of EV71 virus was expressed and purified in vitro by Promega Company, with a purity of >85%.
[0132] 1.3 Reagents
[0133] The antiviral compounds ribavirin, oseltamivir, peramivir, favipiravir and rupintravir were all purchased from Bailingwei Technology Co., Ltd., and the luciferin detection kit Bright-Glo TM Purchased from Promega Company, the rest of the reagents were d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com