A mutant that improves the thermal stability of heparanase I based on molecular dynamics analysis of enzyme flexibility and its preparation method
A thermostability and mutant technology, applied in the fields of genetic engineering and protein expression, can solve the problems of heavy workload and achieve the effects of extending shelf life, reducing production costs, and reducing screening workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0038] The present invention will be described in further detail below in conjunction with specific examples. The following examples are only descriptive, not restrictive, and cannot limit the protection scope of the present invention.
[0039] The raw materials used in the present invention, unless otherwise specified, are conventional commercially available products; the methods used in the present invention, unless otherwise specified, are conventional methods in the art.
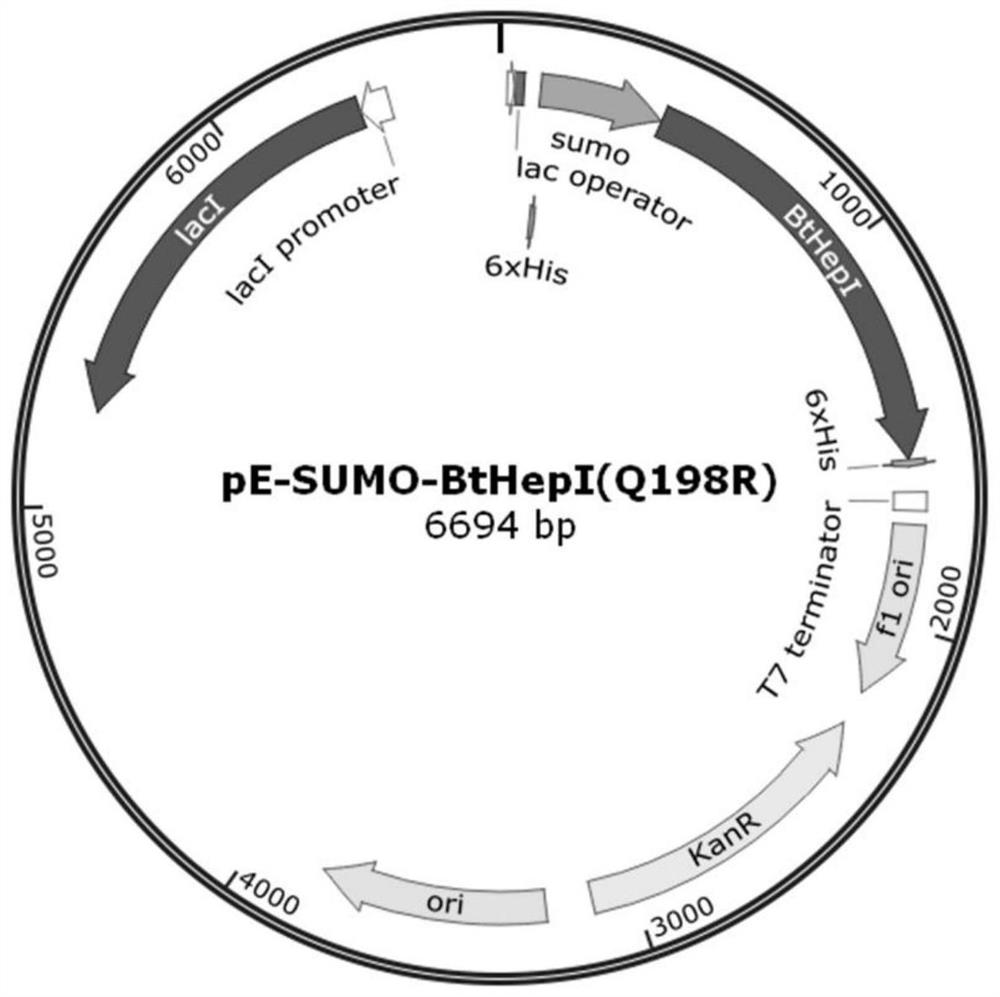
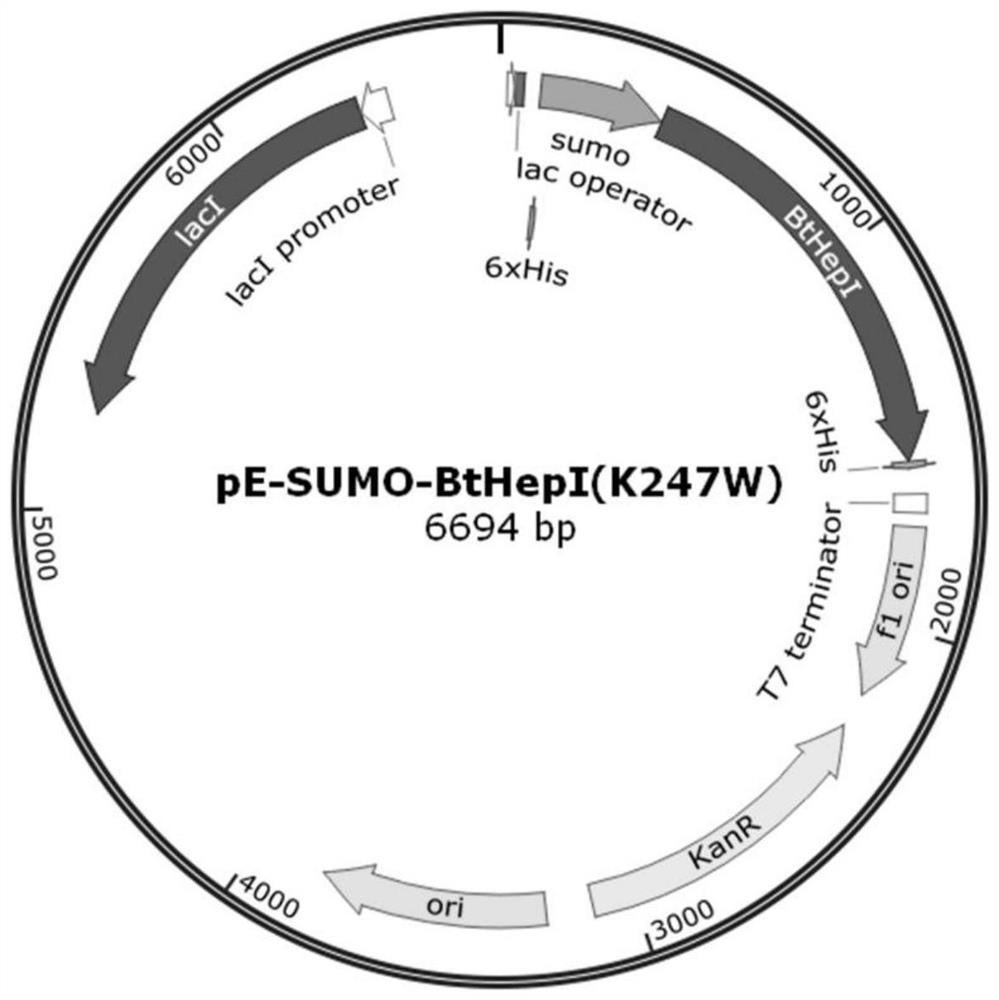
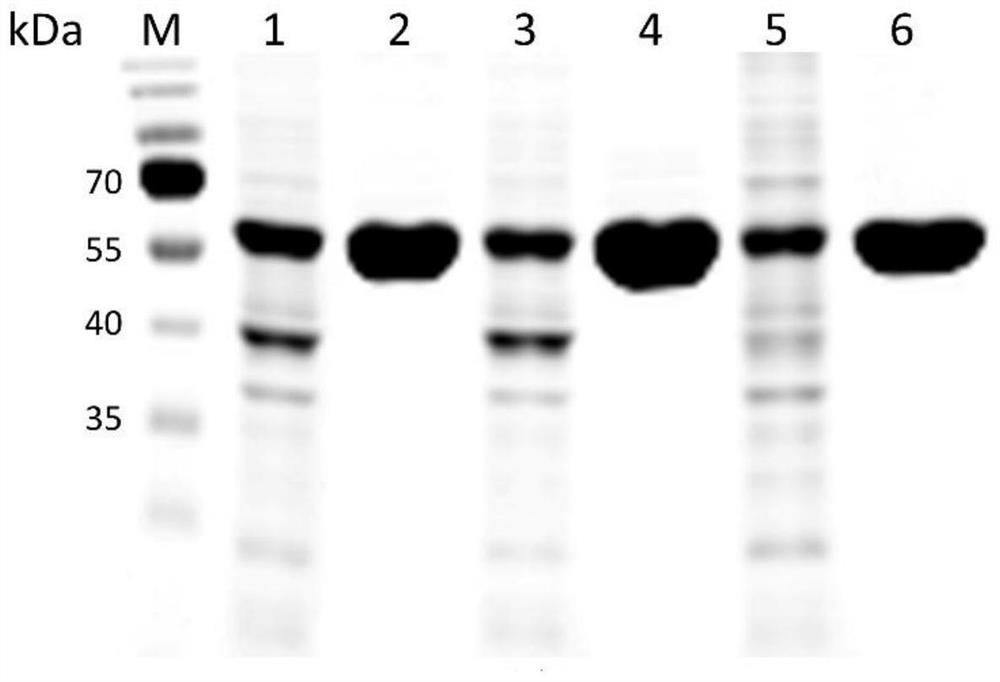
[0040] A mutant that improves the thermal stability of heparanase I based on molecular dynamics analysis of enzyme flexibility, the mutant is mutant BtHepI Q198R and / or mutant BtHepI K247W , the mutant BtHepI Q198R The amino acid sequence is SEQID NO.1, the mutant BtHepI K247W The amino acid sequence of is SEQ ID NO.2.
[0041] An encoding gene of a mutant that improves the thermostability of heparanase I based on molecular dynamics analysis of enzyme flexibility as described above, the mutant BtHepI ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com