Asymmetric constrained-geometry dinuclear metallocene compound and preparation method and application thereof
A metallocene compound, asymmetric technology, applied in the direction of titanium organic compounds, chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, etc. problem, to achieve the effect of stable steric configuration, good catalytic activity and copolymerization ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] The present embodiment provides a kind of asymmetric restricted configuration dinuclear metallocene compound, the molecular formula of this compound (complex 1) is: [(tBuNTiCl 2 )(η 5 -C 5 h 4 )C(H)(CH 2 ) 2 C(CH 3 )(η 5 -C 5 h 4 )(tBuNTiCl 2 )].
[0080] The synthetic route of complex 1 is as follows:
[0081]
[0082] The specific preparation process of complex 1 is:
[0083] (1) Under the protection of argon, use methanol as a solvent, mix 4-oxopentaldehyde (100g, 1.0mol) and tetrahydropyrrolidine (7.0g, 0.10mol), cool the reaction bottle to 0°C, and slowly drop Add the newly cracked cyclopentadiene (132g, 2.0mol), continue to stir the reaction for 4 hours after the completion of the dropwise addition, add the saturated aqueous common salt solution of dilute acetic acid after the completion of the reaction to adjust the pH to weak acidity, remove the solvent methanol on the rotary evaporator, The aqueous phase was extracted with ether, and the combined...
Embodiment 2
[0089] This example provides the application of the complex 1 prepared in Example 1 as a catalyst in homogeneously catalyzed ethylene polymerization.
[0090] Homogeneously catalyzed ethylene polymerization comprises the following steps:
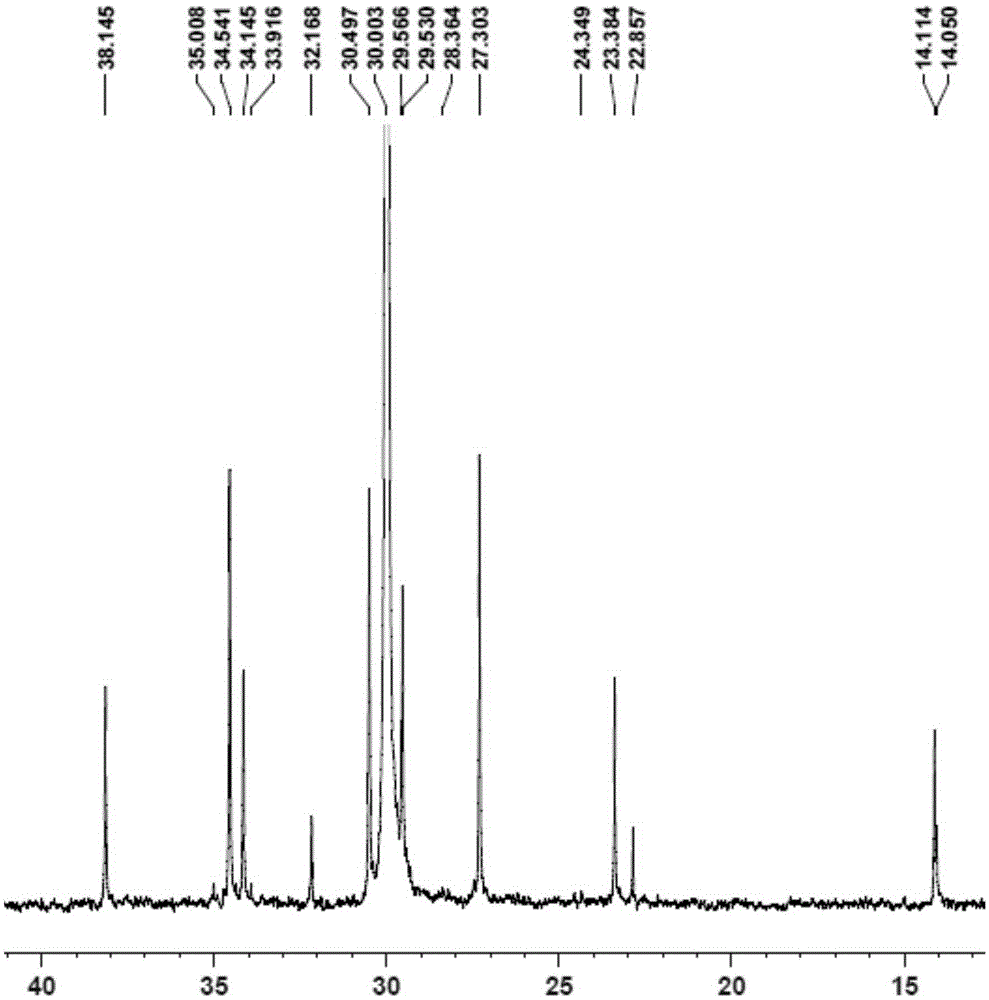
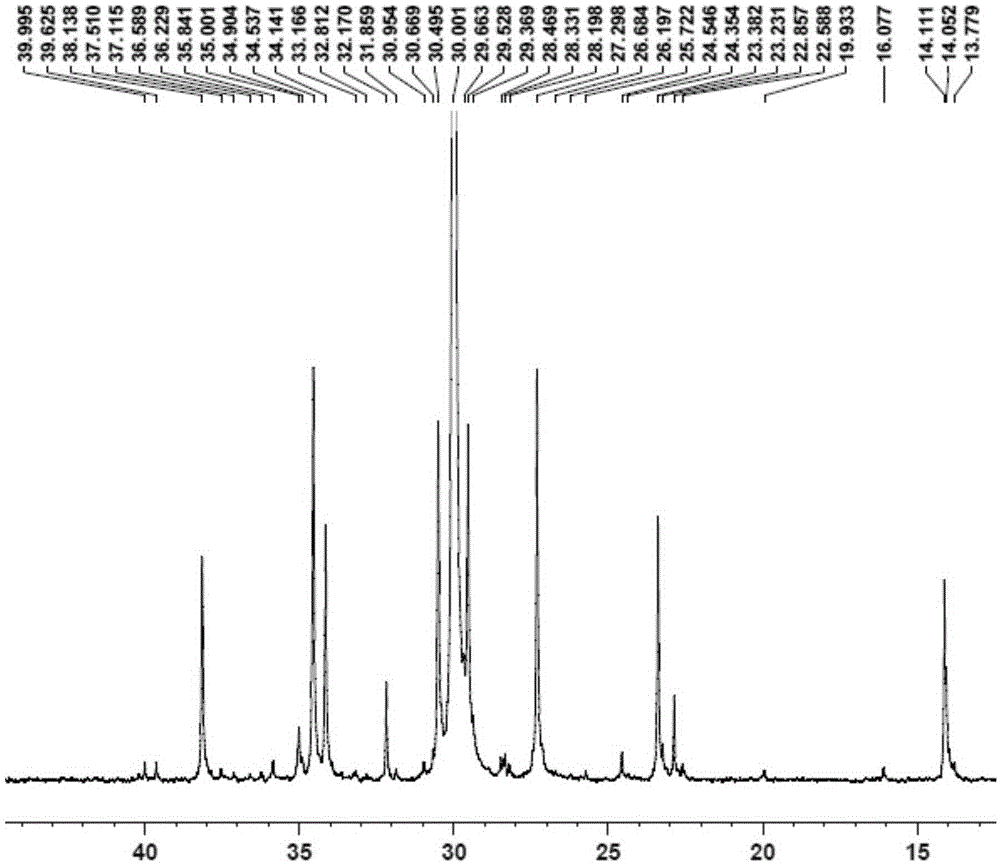
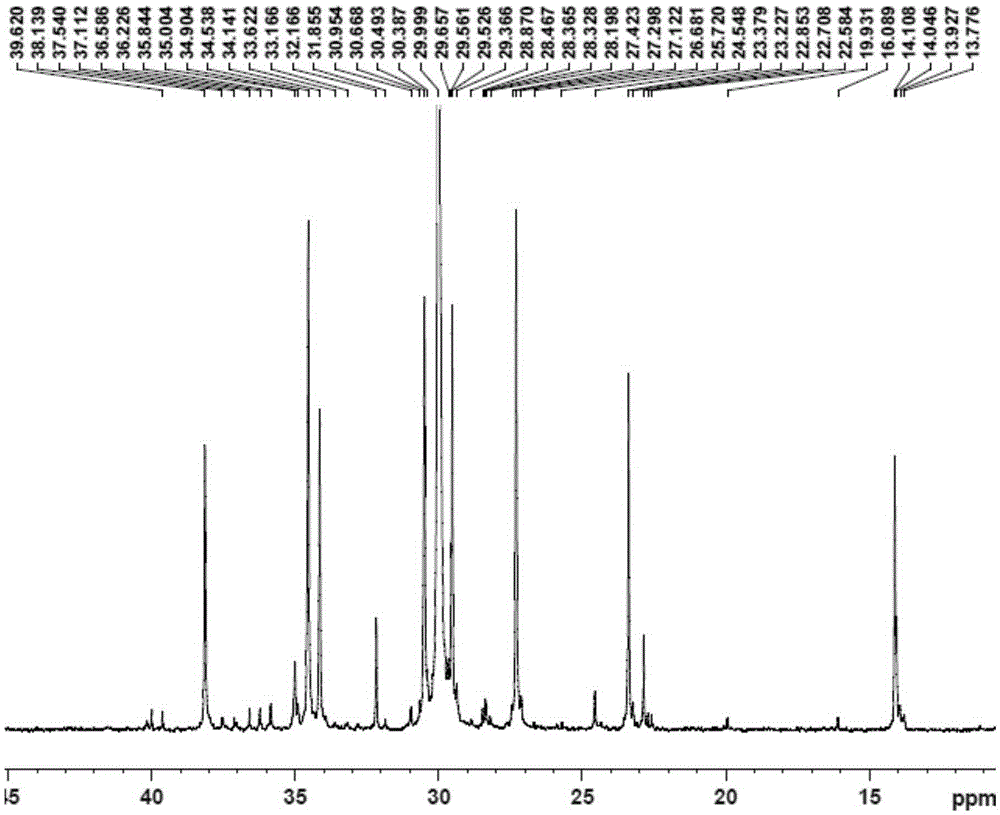
[0091] Replace the 100mL autoclave equipped with a magnetic stirrer and air duct with ethylene gas for 3 times, under the protection of nitrogen, add toluene, cocatalyst MAO2.5mL (1.60M)[Al / M=1000], 2.0μmol complexation Material 1, control the total volume to 100mL, feed ethylene gas, start the polymerization reaction at 50°C, maintain the ethylene pressure at 1.2MPa, stir the reaction for 30min, close the cylinder, release the pressure, and then terminate the reaction with 10% hydrochloric acid ethanol. Transfer the polymer to a beaker, let it stand overnight, filter and wash the polymer fully with ethanol, dry it in vacuum at 50°C to constant weight, weigh the polymer mass, and calculate that the polymerization activity of the catalyst is ...
Embodiment 3
[0093] This example provides the application of the complex 1 prepared in Example 1 as a catalyst in the homogeneously catalyzed copolymerization of ethylene and 1-hexene.
[0094] Homogeneously catalyzed ethylene and 1-hexene copolymerization comprises the following steps:
[0095] Replace the 100mL autoclave equipped with a magnetic stirrer and gas tube with ethylene gas for 3 times, and add toluene, 1-hexene 10mL, cocatalyst MAO2.5mL (1.60M) [Al / M=1000 ], 2.0μmol complex 1, control the total volume to 100mL, feed ethylene gas, start the polymerization reaction at 50°C, maintain the ethylene pressure at 0.3MPa, stir the reaction for 30min, close the cylinder, and terminate the reaction with 10% hydrochloric acid ethanol . Transfer the polymer to a beaker, let it stand overnight, filter and wash the polymer fully with ethanol, dry it in vacuum at 50°C to constant weight, weigh the polymer, and calculate the catalyst polymerization activity to be 1.14×10 6 gpolymer / molM h, M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com