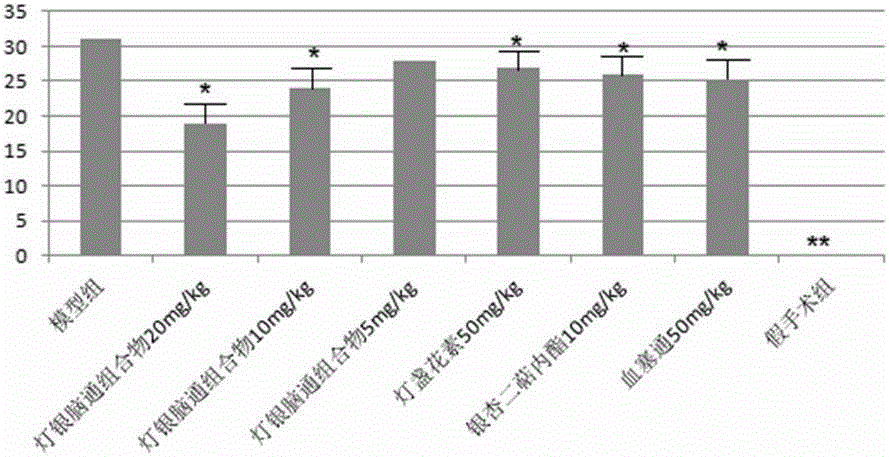
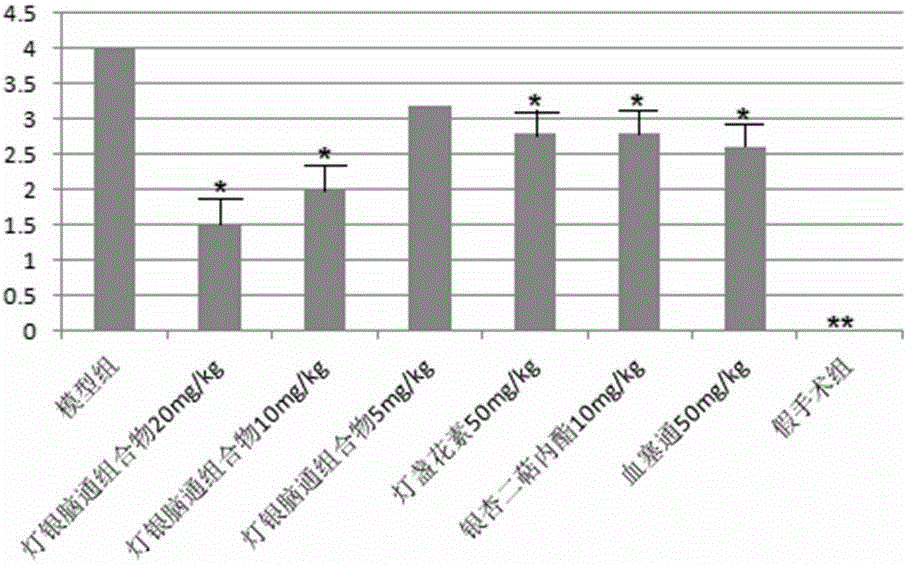
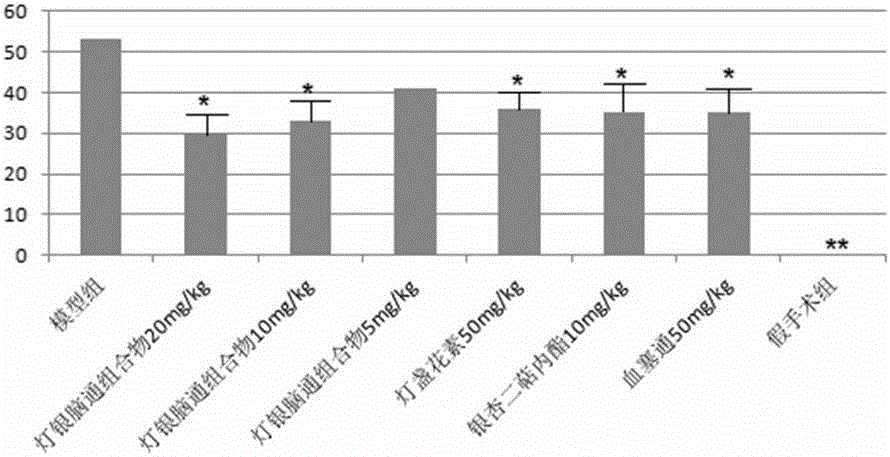
Dengyin naotong pharmaceutical composition and preparation method thereof, preparation and application thereof
A lamp-yin-nao-tong and composition technology, which is applied in the field of medicine, can solve the problems of inability to guarantee product quality and safety, inability to quickly and fully exert the efficacy of drugs, and inability to make chemical components, so as to reduce pathological changes and behavioral disorders, Fast-acting and powerful effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The preparation method of the Dengyinnaotong pharmaceutical composition of the present invention includes the steps of pretreatment, preparation and posttreatment, specifically including:
[0036] A. Pre-processing:
[0037] 1) Add a co-solvent with a mass volume ratio of 1:10~100 to the ginkgolide B in the formula ratio to dissolve to obtain a solution a;
[0038] 2) Dissolve the ginkgolide B weight ratio of ginkgolide B in the solubilizer with a weight ratio of 1:30~200 into water with a mass volume ratio of the solubilizer of 1:2~5 to obtain solution b;
[0039] 3) Add solution a dropwise to solution b, and stir at 20-80°C for 1-6 hours until no co-solvent remains to obtain solution c;
[0040] 4) Dissolve the scutellarin molar ratio of 1:0.95~1.05 in the base with alkali mass volume ratio of 1:50~500 to dissolve the solution d, then add the scutellarin in the formula ratio In solution d, adjust the pH value to 6.5~7.2 to obtain solution e;
[0041] B. Preparation...
Embodiment 1
[0061] Example 1—Preparation of freeze-dried powder injection of Dengyinnaotong pharmaceutical composition of the present invention
[0062] Prescription composition:
[0063] Scutellarin 23g
[0064] Ginkgolide B6g
[0065] Ginsenoside Rg126g
[0066] Ginsenoside Rb145g
[0067] Hydroxypropyl Beta Cyclodextrin 480g
[0068] Meglumine 10g
[0069] Water for injection 2000ml appropriate amount (finally removed)
[0070] Preparation method: Dissolve the prescription amount of ginkgolide B with 20 times (mass / volume ratio, mg / ml) acetone, and slowly add the solution to 30% (mass / volume ratio, mg / ml) prescription amount of hydroxyl Propyl β-cyclodextrin aqueous solution, and stirred at 60°C for 6 hours until no co-solvent remained. Dissolve the prescribed amount of scutellarin with the prescribed amount of meglumine plus an appropriate amount of aqueous solution until the pH of the solution is 6.8 for use. Mix the scutellarin solution with the ginkgolide solution, then wei...
Embodiment 2
[0071] Example 2—Preparation of freeze-dried powder injection of Dengyinnaotong pharmaceutical composition of the present invention
[0072] Prescription composition:
[0073] Scutellarin 10g
[0074] Ginkgolide B5g
[0075] Ginsenoside Rg155g
[0076] Ginsenoside Rb130g
[0077] Hydroxypropyl Beta Cyclodextrin 420g
[0078] Meglumine 4g
[0079] Cysteine Hydrochloride 1g
[0080] Water for injection 2000ml appropriate amount (finally removed)
[0081] Preparation method: dissolve the prescribed amount of ginkgolide B with 20 times (mass / volume ratio, mg / ml) ethyl acetate, and slowly add the solution to 30% (mass / volume ratio, mg / ml) of the prescribed amount Hydroxypropyl β-cyclodextrin aqueous solution, and stirred at 40°C for 6 hours until no co-solvent remained. Dissolve the prescribed amount of scutellarin with the prescribed amount of meglumine plus an appropriate amount of aqueous solution until the pH of the solution is 7.2 for use. Mix the scutellarin soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com