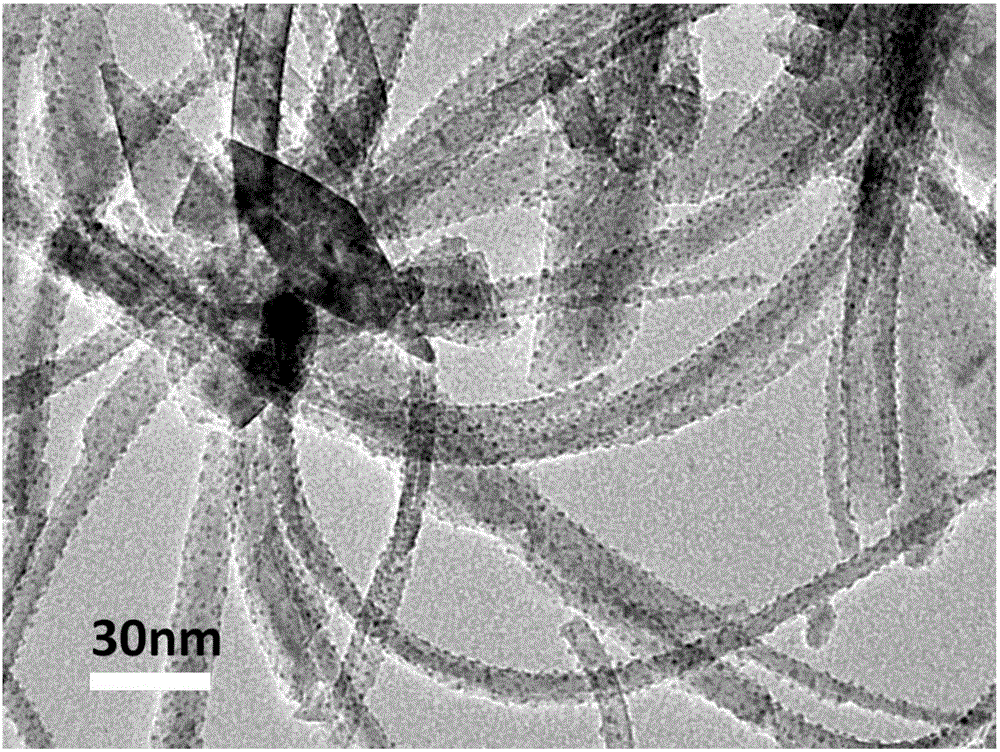
Room-temperature formaldehyde decomposing agent suitable for air purifier and preparation method thereof
An air purifier and formaldehyde decomposition technology, applied in separation methods, chemical instruments and methods, and dispersed particle separation, can solve problems such as limited adsorption, pollution, and limited catalytic efficiency, and achieve efficient capture, efficient indoor formaldehyde pollution, and The effect of solving indoor formaldehyde pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Weigh 0.28g of isopropyl titanate, disperse in 13.8g of concentrated hydrochloric acid, stir and mix for 10 minutes to obtain isopropyl titanate hydrochloric acid solution; weigh 1g of diatomaceous earth and mix it into the aforementioned isopropyl titanate In the hydrochloric acid solution, fully stir and disperse evenly, and the obtained mixed solution is named mixed solution 1;
[0021] (2) Weigh 0.22g cetyltrimethylammonium bromide and dissolve it in 27.3ml deionized water to make a 0.8wt% aqueous solution, then add it dropwise to mixed solution 1 to obtain solution A; then weigh urea 1.96 g was added into solution A, stirred for 20 minutes, and the mixed solution was named as mixed solution 2, and its total volume was 40ml;
[0022] (3) Measure 120ml of ethylene glycol, mix and stir with the mixed solution 2 for 10 minutes, and the obtained mixed solution is named as the precursor solution 3;
[0023] (4) Transfer the obtained precursor solution 3 to a hydroth...
Embodiment 2
[0028] (1) Weigh 0.42g of isopropyl titanate, disperse in 13.8g of concentrated hydrochloric acid, stir and mix for 10 minutes to obtain isopropyl titanate hydrochloric acid solution; weigh 1g of diatomaceous earth and mix it into the aforementioned isopropyl titanate In the hydrochloric acid solution, fully stir and disperse evenly, and the obtained mixed solution is named mixed solution 1;
[0029] (2) Weigh 0.22g cetyltrimethylammonium bromide and dissolve it in 27.3ml deionized water to make a 0.8wt% aqueous solution, then add it dropwise to mixed solution 1 to obtain solution A; then weigh urea 1.96 g was added into solution A, stirred for 20 minutes, and the mixed solution was named as mixed solution 2, and its total volume was 40ml;
[0030] (3) Measure 120ml of ethylene glycol, mix and stir with the mixed solution 2 for 10 minutes, and the obtained mixed solution is named as the precursor solution 3;
[0031] (4) Transfer the obtained precursor solution 3 to a hydroth...
Embodiment 3
[0035] (1) Weigh 0.28g of isopropyl titanate, disperse in 13.8g of concentrated hydrochloric acid, stir and mix for 20 minutes to obtain isopropyl titanate hydrochloric acid solution; weigh 0.5g of diatomaceous earth and mix it into the aforementioned isopropyl titanate In the ester hydrochloric acid solution, fully stir and disperse evenly, and the obtained mixed solution is named mixed solution 1;
[0036] (2) Weigh 0.22g cetyltrimethylammonium bromide and dissolve it in 27.3ml deionized water to make a 0.8wt% aqueous solution, then add it dropwise to mixed solution 1 to obtain solution A; then weigh urea 1.96 g was added into solution A, stirred for 20 minutes, and the mixed solution was named as mixed solution 2, and its total volume was 40ml;
[0037] (3) Measure 120ml of ethylene glycol, mix and stir with the mixed solution 2 for 20 minutes, and the obtained mixed solution is named as the precursor solution 3;
[0038] (4) Transfer the obtained precursor solution 3 to a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com