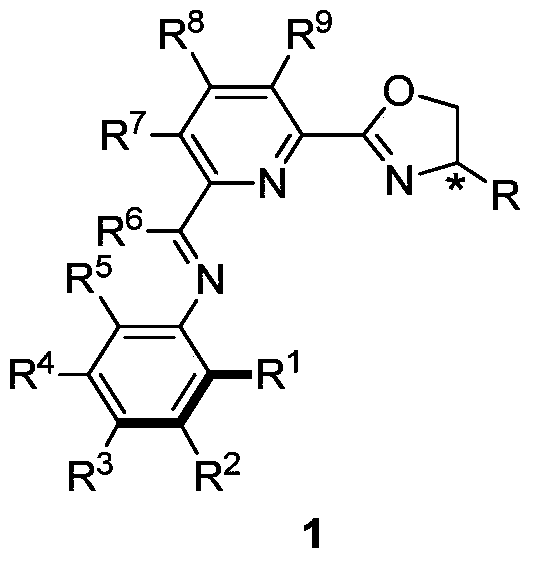
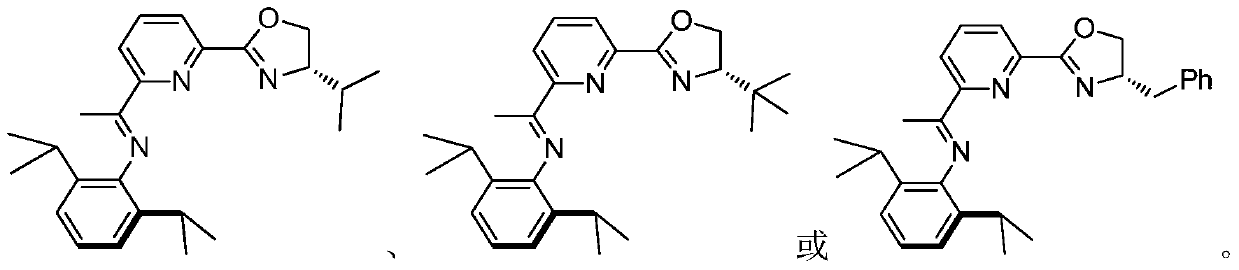
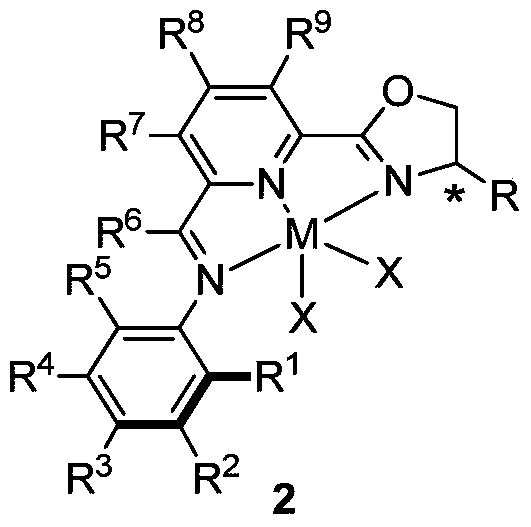
nnn ligand, its metal complex, preparation method and application
A technology of metal complexes and ligands, applied in the direction of iron organic compounds, cobalt organic compounds, chemical instruments and methods, etc., can solve the problems of limited enantioselectivity and poor enantioselectivity, and achieve cheap and easy-to-obtain raw materials and mild reaction conditions , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Embodiment 1: Preparation of NNN ligand 1 according to the present invention
[0112]
[0113] Compound 4a: 2-cyano-6-acetylpyridine (2.00g, 13.68mmol), 2,6-diisopropylaniline (2.91g, 16.42mmol), p-methylbenzenesulfonate were added to a 100mL three-necked flask Acid monohydrate (130mg, 0.68mmol), and solvent toluene 35mL, equipped with a reflux device, heated the reaction to reflux for 48h. Cool to room temperature, concentrate in vacuo, and purify by flash column chromatography (ethyl acetate:petroleum ether=1:30) to obtain a yellow solid (4.05 g, 97%), with a purity of >97% by HS. 1 H NMR (400MHz, CDCl 3 )δ8.60(d, J=7.7Hz, 1H), 7.94(t, J=7.4Hz, 1H), 7.80(d, J=7.5Hz, 1H), 7.23–7.16(m, 2H), 7.12( dd,J=8.6,6.4Hz,1H),2.71–2.64(m,2H),2.22(s,3H),1.16(d,J=5.1Hz,6H),1.14(d,J=5.2Hz,6H ). 13 C NMR (101MHz, CDCl 3 )δ165.94, 157.70, 145.95, 137.59, 135.62, 132.85, 129.48, 124.60, 124.15, 123.20, 117.36, 28.45, 23.32, 22.94, 17.20. Elemental analysis, theoretical value: (C...
Embodiment 2
[0126] Embodiment 2: Preparation of NNN ligand complex 2 of the present invention
[0127]
[0128] (S)-( iPr NNN iPr ) CoCl 2 (complex 2A): in N 2 In the glove box, the CoCl 2 (260mg, 2.0mmol) slowly added to iPr NNN iPr In the yellow solution of 1A (783mg, 2.0mmol) in THF (30mL), the color of the reaction solution gradually changed to yellow-green. After the reaction was stirred at room temperature for 10 h, the mixture was concentrated by an oil pump to obtain a solid, which was washed with an appropriate amount of ether, filtered, and dried in vacuo to obtain a yellow-green solid (995 mg, 95%), with a purity of >97% by HS. Elemental analysis theoretical value. (C 25 h 33 Cl 2 CoN 3 O): C, 57.59; H, 6.38; N, 8.06. Found: C, 57.16; H, 6.45; N, 7.66.
[0129]
[0130] (R)-( iPr NNNiPr ) CoCl 2 (complex 2A-R): in N 2 In the glove box, the CoCl 2 (79mg, 0.61mmol) slowly added to iPr NNN iPr In the yellow solution of 1A-R (250mg, 0.64mmol) in THF (30mL), t...
Embodiment 3
[0141] Embodiment 3: Preparation of the metal complex 3 of the NNN ligand of the present invention
[0142]
[0143] (S)-( iPr NNN iPr ) CoCH 3 (complex 3A): in N 2 In the glove box, place (S)-( iPr Chiral (iminopyridine) oxazoline NNN iPr ) CoCl 2 (80mg, 0.154mmol) in n-pentane (8mL) was cooled to -35°C, and then MeLi (104μL, 0.31mmol; 3.0M in diethoxymethane) was slowly added dropwise at this temperature, and the reaction solution rapidly became deep red. After the dropwise addition, the reaction was reacted at room temperature for 2 h, filtered through celite, and the filtrate was dried by an oil pump to obtain a deep red solid (68 mg, 96%), with a purity of >97% in HS spectrum. 1 H NMR (400MHz, C 6 D. 6 )δ10.29(t, J=7.5Hz, 1H), 8.62(d, J=7.3Hz, 1H), 7.80(t, J=7.5Hz, 1H), 7.71(d, J=7.4Hz, 1H) ,7.66(d,J=7.4Hz,1H), 7.08(d,J=7.7Hz,1H),4.95(dd,J=8.0,4.0Hz,1H),4.77–4.71(m,1H),4.63( t,J=8.8Hz,1H),3.51–3.41(m,1H),3.19–3.06(m,1H),1.46(d,J=2.7Hz,1H),1.30(d,J=6.6Hz,3H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com