Aryl-alkyne compound as well as preparation method and application thereof
A compound and synthesis method technology, applied in chemical instruments and methods, organic chemistry, instruments, etc., can solve problems such as peripheral nervous system injury, environmental pollution, gold loss, etc., achieve high sensitivity and selectivity, simple detection method, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of Aryl-Alkyne Compounds
[0029] The synthetic route of the aryl-alkyne compound (2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-8-yl3-phenylpropiolate) of the present invention is:
[0030]
[0031] The synthetic method of aryl-alkyne compound of the present invention is:
[0032] 8-Hydroxyjulolidine (1mmol) and phenylpropiolic acid (1mmol) were dissolved in dichloromethane (10mL), and the resulting mixture was stirred (60min) at 0°C; then the condensing agent EDC (1-( 3-dimethylaminopropyl)-3-ethylcarbodiimide) 1.1mmol and base DMAP (4-dimethylaminopyridine) 0.5mmol, after stirring at room temperature (25°C) for 5 hours, the reaction was stopped, and at room temperature Concentrate by rotary evaporation, the obtained concentrated solution is separated by 300 mesh silica gel column chromatography, and then rinsed with a mixture of ethyl acetate and petroleum ether with a volume ratio of 1:4, and the rinse solution is concentrated under re...
Embodiment 2
[0037] Embodiment 2: Trivalent gold concentration determination
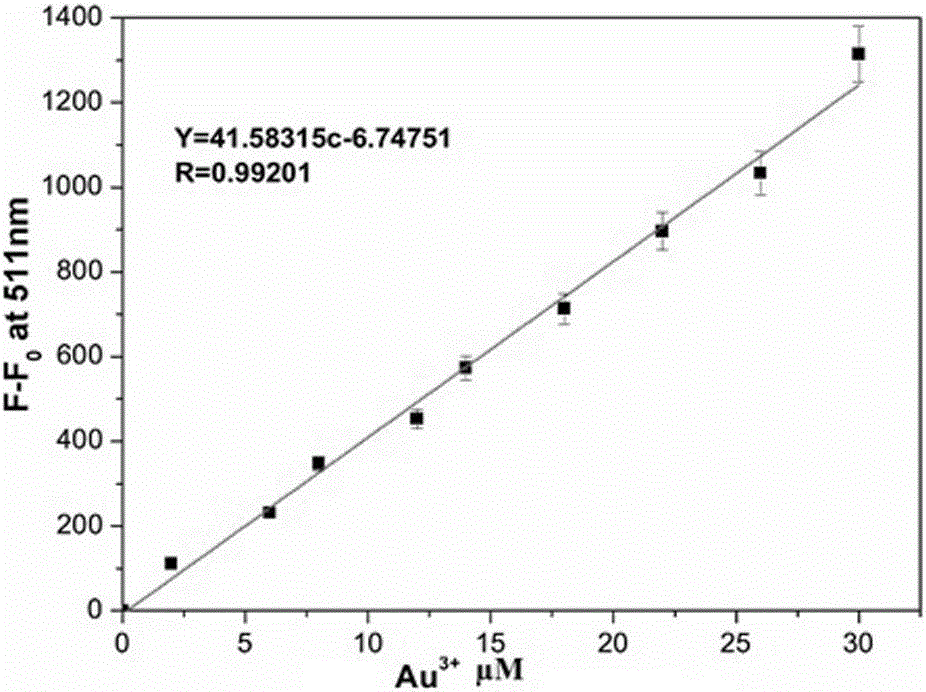
[0038] (1) Establish a trivalent gold detection regression equation
[0039] (1) Prepare a HEPES buffer solution with a pH of 7.4 and a concentration of 10 mM; prepare a 2 mM methanol solution of an aryl-alkyne compound;
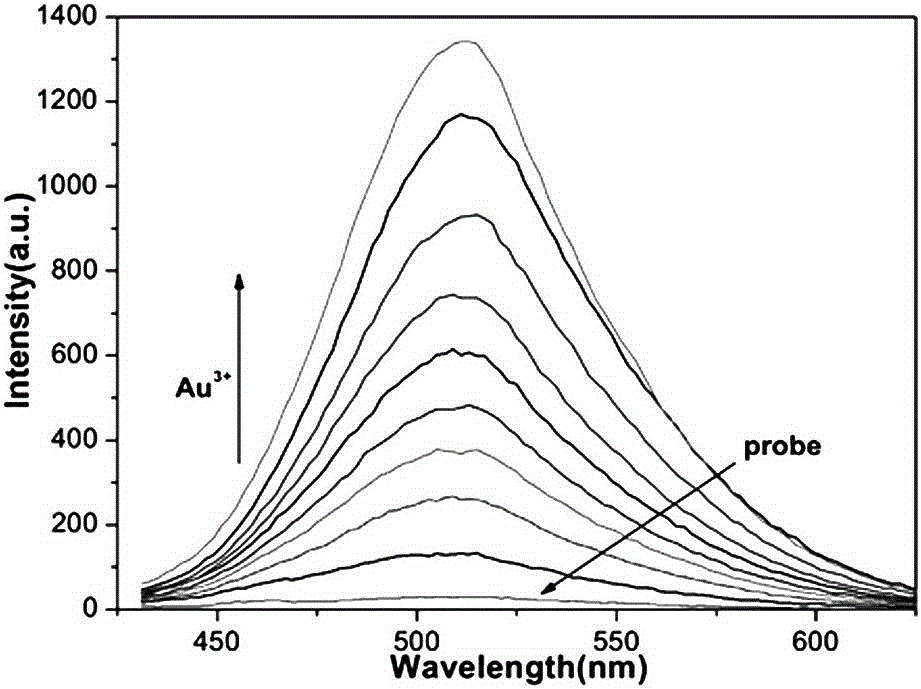
[0040] (2) According to the volume ratio of 20000:1, add 2000 μL of HEPES buffer solution and 0.1 μL of methanol solution of aryl-alkyne compound into a clean fluorescence cuvette, and detect its fluorescence emission curve ( figure 1 The curve referred to in the probe), which detects the fluorescence intensity value F at 511nm on the fluorescence spectrophotometer 0 =27;
[0041] (3) Add 2000 μL of HEPES buffer solution and 0.1 μL of methanol solution of aryl-alkyne compound to another fluorescent cuvette, and then gradually add standard Au 3+ Aqueous solution (Au 3+ The concentration is 2mmol / L), when the accumulatively added standard Au 3+ When the volume of the aqueous solution is 2 μ...
Embodiment 3
[0048] Embodiment 3: Anti-interference test
[0049] Prepare a HEPES buffer solution with a pH of 7.4 and a concentration of 10 mM, and prepare a 2 mM solution of aryl-alkyne compound with methanol; add 2 mL of HEPES buffer solution and 0.1 µL of the aryl-alkyne compound in methanol;
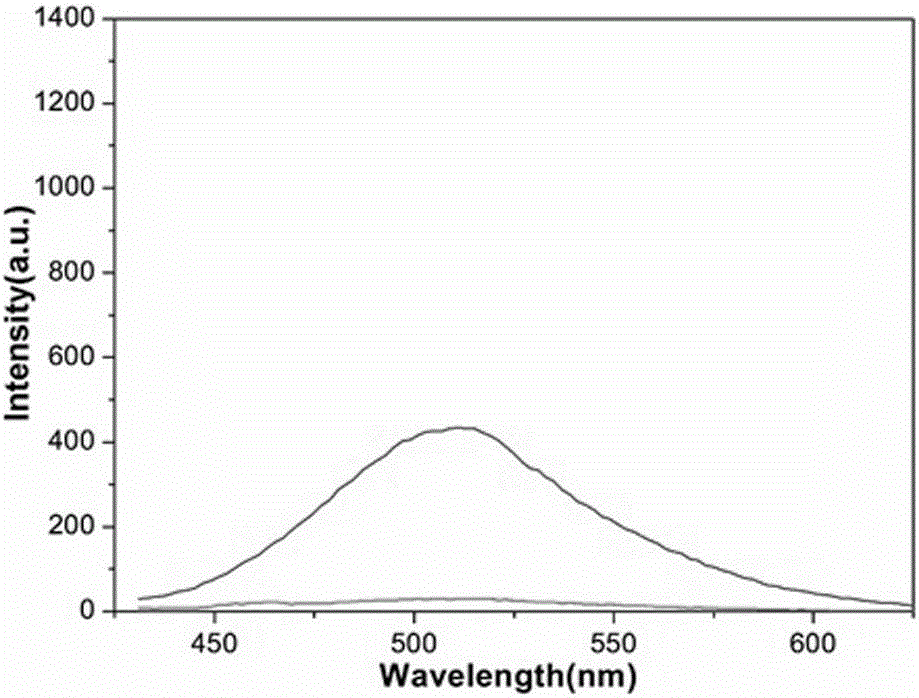
[0050] Add no other metal ion solutions to the number 1 cuvette; add 2 molar equivalents of Au to the number 2 cuvette 3+ Aqueous solution, 40 molar equivalents of 19 kinds of common cation aqueous solutions were added to the numbered 3-20 cuvettes (in order of Hg 2+ , Mg 2+ , Ca 2+ 、Cu 2+ , Fe 3+ , Zn 2+ 、Ni 2+ 、 Bi 3+ 、Co 2+ 、Vo 2+ , Mn 2+ 、Ba 2+ 、Cd 2+ , Pb 2+ , Sn 2+ , Yb 3+ 、Cr 3+ , La 3+ 、Er 3+ ), and then put the 20 cuvettes on a fluorescence spectrophotometer to detect their fluorescence intensity values at 511nm, and draw a histogram of the relative fluorescence intensity at 511nm corresponding to different cations, as Figure 4 shown.
[0051] Figure 4 The resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com