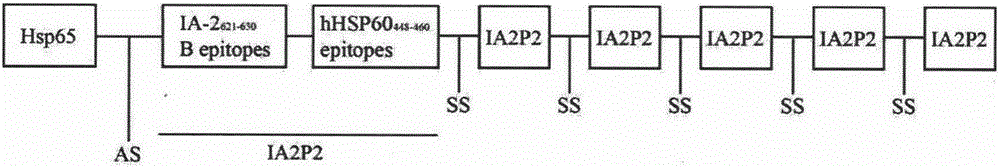
Novel recombinant lactobacillus resisting diabetes mellitus type 1 and application thereof
A technology for recombining lactic acid bacteria and Lactococcus lactis, applied in the field of biomedicine, can solve the problem of high safety production cost, save the purification process, realize large-scale production, and achieve good therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
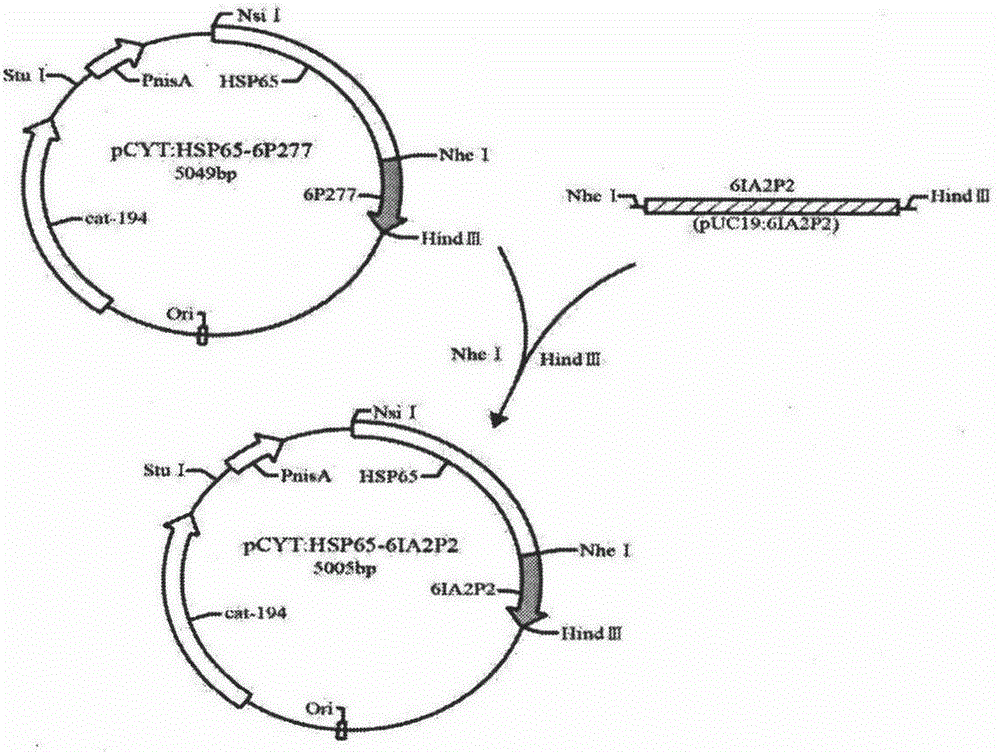
[0054] The cloning of embodiment 1HSP65-6IA2P2 gene
[0055] The universal primers M13F: 5'-TGTAAAACGACGGCCAGT-3' and M13R: 5'-CAGGAAACAGCTATGACC-3' located on both sides of the target gene on the vector were used as upstream and downstream primers respectively, and the two primers were synthesized by Shanghai Sangon Bioengineering Co., Ltd. The PUC19 plasmid was used as a template for PCR, and the gene fragment encoding 6IA2P2 sequence was obtained. Then it was digested with NheI and HindIII, and connected into the PCYT:HSP65-6P277 vector which was also digested with NheI and HindIII, to construct PCYT:HSP65-6IA2P2.
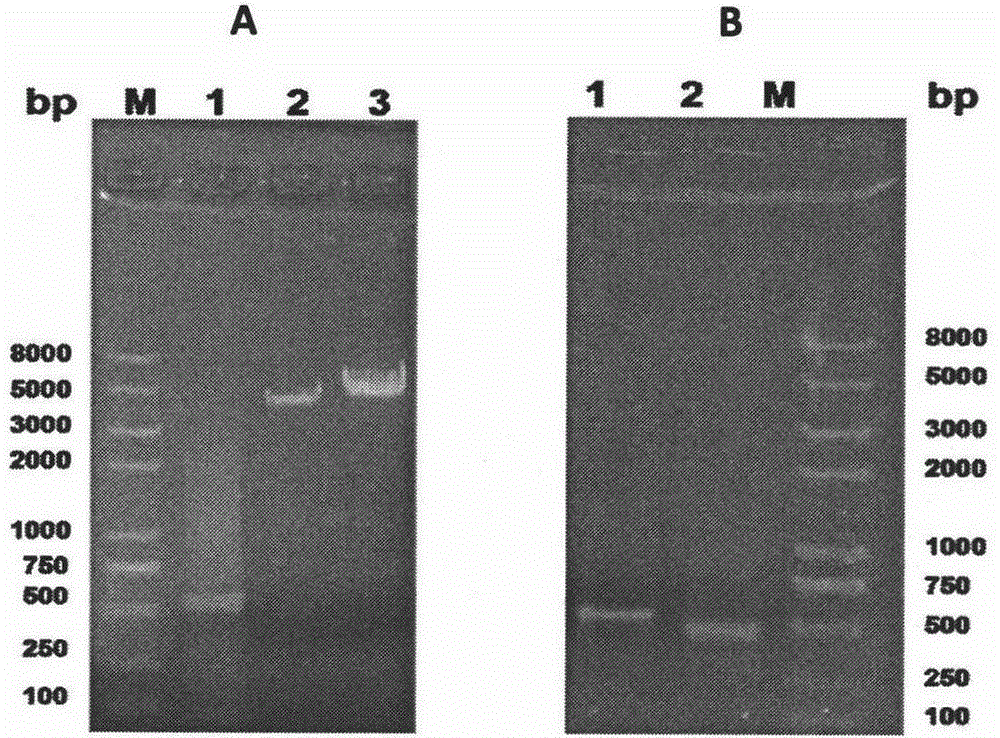
[0056] The PCR reaction was carried out according to the following conditions: in each step of the reaction, the upper and lower primers were mixed at a ratio of 1:1, and the reaction program was set to 94°C, 1s; 50°C, 45s; 72°C, 50s; a total of 30 cycles, 0.8% agarose gelation PCR products were identified by gel electrophoresis (see image 3 ), the band posit...
Embodiment 2
[0058] Example 2 The recombinant plasmid PCYT: HSP65-6IA2P2 was transformed into Lactococcus lactis competent cells by electric shock transformation
[0059] The process of preparing Lactococcus lactis competent cells is roughly as follows: pick a single colony from a fresh GM17 agar plate, inoculate 10 mL of GM17 medium, and culture overnight at 30°C. Then inoculate 1 mL of the overnight culture into 50 mL of preheated GM17 medium containing 1-2.5% glycine, and culture at 30°C. Monitor OD 600nm. When the OD value of the culture is close to 0.2-0.7, take out the culture and cool it on ice for 10min-30min, then centrifuge the culture in a sterilized tube, centrifuge at 5000g at 4°C for 15min, discard the supernatant, and use an equal volume of ice pre-cooled Suspend the cell pellet twice in OSPS buffer and resuspend the cells with 1 / 100 OSPS. Finally, the cells were added to a sterilized 1.5mL centrifuge tube, snap-frozen in a dry ice-ethanol bath, and stored at -70°C or use...
Embodiment 3
[0060] Example 3 Induction of recombinant Lactococcus lactis strain CCTCCNO: M2014609 to express fusion protein HSP65-6IA2P2
[0061] The recombinant Lactococcus lactis CCTCCNO: M2014609 obtained in Example 2 was inoculated in GM17 medium and cultured overnight at 30°C; the overnight culture solution was transferred to GM17 medium at a ratio of 1:100 and expanded at 30°C until the bacteria grew to an OD value of 0.4 At ~0.7, add the inducer Nisin, continue to cultivate for a period of time, and harvest the bacteria to prepare protein samples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com