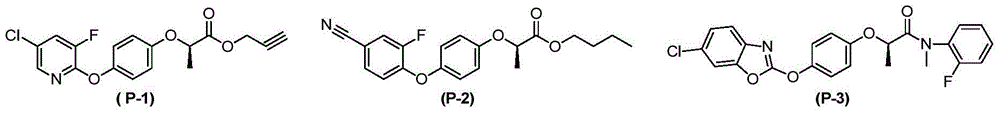
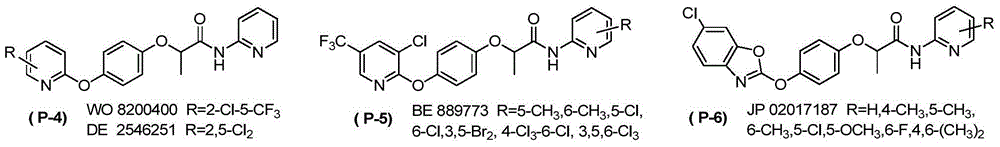
N-pyridine aryloxyphenoxy carboxylic acid derivatives, preparation method and applications thereof
A technology of pyridine aryloxyphenoxycarboxylic acid and derivatives, which can be used in the fields of herbicide, insecticide/acaricide, and sterilization, and can solve the problems of unsatisfactory rice safety and inability to control weeds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Example 1 This example illustrates the preparation of compound 01(R / S) in Table 1.
[0189]
[0190] (R / S)-2-(4-Hydroxyphenoxy)propionic acid Add 2-bromopropionic acid to 50% NaOH (0.1mol) solution at 15-25°C, stir to dissolve and add 30mL of water , continue to stir for 5-10 minutes, set aside. Under the condition of 15-25°C, add hydroquinone (0.4mol) and 50mL of water into the reaction bottle, add 50% NaOH (0.8mol) under stirring, then add the NaOH solution of the above-mentioned 2-bromopropionic acid, 50-60 React at ℃ for 4-6 hours, cool, adjust the pH to 4.0-4.5 with dilute hydrochloric acid, extract with ethyl acetate, adjust the pH to 1.0-1.5 with dilute hydrochloric acid, extract with isopropyl acetate, add water to the organic phase to azeotropically remove isopropyl acetate, Adjust the pH of the aqueous solution to 2.0-2.5 with 50% NaOH solution, cool, extract with ethyl acetate, dry over anhydrous sodium sulfate, and remove the solvent to obtain (R / S)-2-(4...
Embodiment 2
[0195] Example 2 This example illustrates the preparation of compound 02(R) in Table 1.
[0196]
[0197] (R)-2-(4-((5-Chloro-3-fluoropyridin-2-yl)oxy)phenoxy)propanoic acid was replaced by (R)2-(4-hydroxyphenoxy)propanoic acid (R / S) 2-(4-hydroxyphenoxy)propionic acid, synthesize (R)-2-(4-((5-chloro-3-fluoropyridin-2-yl) oxygen with reference to the method of Example 1 base) phenoxy) propionic acid.
[0198] (R)-N-(3-nitropyridin-2-yl)-2-(4-((3-fluoro-5-chloropyridin-2-yl)oxy)phenoxy)propionamide (R) -2-[4-(3-fluoro-5-chloropyridin-2-yl)oxy)phenoxy]propanoic acid (1.04g, 3.3mmol), dichloromethane (40mL), 2-amino-3- Nitropyridine (3.3mmol), add triethylamine (10mmol) and methanesulfonyl chloride (3.3mmol) dropwise under ice cooling condition, react overnight at room temperature, pour into 100-200mL ice water, extract with dichloromethane, anhydrous sulfuric acid Dry over sodium, remove the solvent, and purify by column chromatography to give 0.30 g of the light yellow tit...
Embodiment 3
[0199] Example 3 This example illustrates the preparation of compound 03(R / S) in Table 1.
[0200]
[0201] 3-nitropyridine-2-methylamine 2-chloro-3-nitropyridine (0.02mol), 25~30% methylamine solution (0.2mol,), potassium carbonate (0.04mol), catalytic amount of potassium iodide, DMF ( 50 mL), react at room temperature for 10-12 h, pour the reaction solution into 200 mL of water, filter, wash with water, and dry to obtain 2.93 g of the title compound as a yellow solid, with a yield of 95.8%.
[0202] (R / S)N-Methyl-N-(3-nitropyridin-2-yl)-2-(4-((3-fluoro-5-chloropyridin-2-yl)oxy)phenoxy ) Propionamide reaction flask was added (R / S)-2-(4-(3-fluoro-5-chloropyridin-2-yloxy)phenoxy)propionyl chloride (3.3mmol), dichloromethane (40mL ), 3-nitropyridine-2-methylamine (3.3mmol) and catalytic amount of 4-dimethylaminopyridine (DMAP), stirred at room temperature for 10min, after dripping triethylamine (10mmol), the temperature was raised to reflux reaction 3 ~5hr, pour into 100~20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com