A kind of method for separating and measuring canagliflozin and related substances
A technology related to substances and organic phases, applied in the direction of material separation, measuring devices, and analyzing materials, etc., to achieve the effects of high theoretical plate number, small baseline noise, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
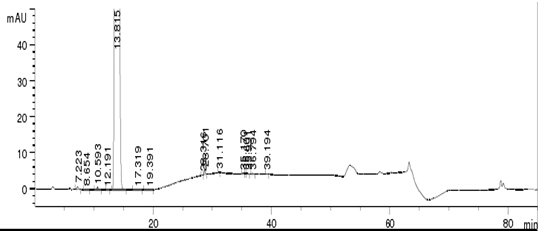
Embodiment 1
[0051] Instruments and Conditions
[0052] High performance liquid chromatography: Agilent 1260;
[0053] Chromatographic column: a chromatographic column filled with butylsilane bonded silica gel (Inertsil-C4, 4.6×250mm, 5µm);
[0054] Mobile phase A: aqueous phosphoric acid solution, take purified water, adjust the pH value to 3.5 with phosphoric acid, mobile phase B: organic phase acetonitrile;
[0055] Elute according to the gradient elution table (see Table 1) (after 50 min, it is the stage of flushing the column to balance the system).
[0056]
[0057] Injection volume: 20μl;
[0058] Column temperature: 25°C;
[0059] Detection wavelength: 210nm.
[0060] Experimental procedure
[0061] Take about 25 mg of canagliflozin raw material, accurately weigh it, put it in a 50 ml volumetric flask, add solvent 70% acetonitrile solution to dissolve and dilute to the mark, shake well, and use it as the test solution. Get solvent and need testing solution, carry out HPLC ...
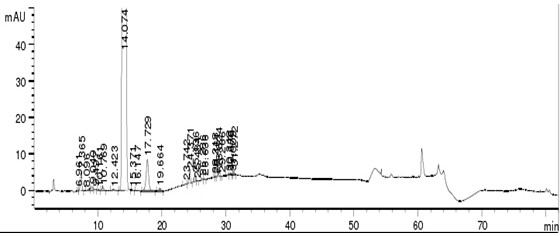
Embodiment 2
[0063] Instruments and Conditions
[0064] High performance liquid chromatography: Agilent 1260;
[0065] Chromatographic column: a chromatographic column filled with butylsilane bonded silica gel (Inertsil-C4, 4.6×250mm, 5µm);
[0066] Mobile phase A: phosphoric acid solution, take purified water, adjust the pH value to 3.5 with phosphoric acid, mobile phase B: acetonitrile;
[0067] Elute according to the gradient elution table (see Table 2) (after 50 min, it is the stage of flushing the column to balance the system).
[0068]
[0069] Injection volume: 20μl;
[0070] Column temperature: 25°C.
[0071] Experimental procedure
[0072] Take about 25 mg of the crude drug of canagliflozin, accurately weigh it, put it in a 50 ml volumetric flask, add solvent 70% acetonitrile solution to dissolve and dilute to the mark, shake well, and use it as the test solution. Get need testing solution, carry out HPLC analysis under above-mentioned chromatographic condition, with 210nm...
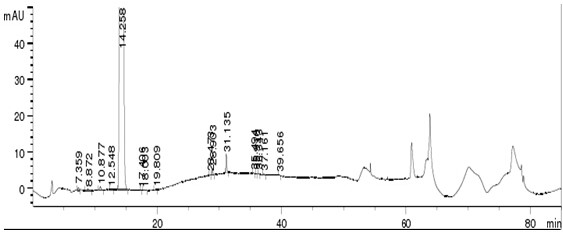
Embodiment 3
[0074] Instruments and Conditions
[0075] High performance liquid chromatography: Agilent 1260;
[0076] Chromatographic column: a chromatographic column filled with butylsilane bonded silica gel (Inertsil-C4, 4.6×250mm, 5µm);
[0077] One set of mobile phase A: phosphoric acid solution, take purified water, use phosphoric acid to adjust the pH value to 2.0, another set of mobile phase A: phosphoric acid solution, take purified water, use phosphoric acid to adjust the pH value to 6.0, mobile phase B: acetonitrile; The mobile phase A of the group was combined with the mobile phase B respectively according to the gradient elution table (see Table 3) and eluted once (after 50 minutes, it was the stage of flushing the column to balance the system).
[0078]
[0079] Injection volume: 20μl;
[0080] Column temperature: 25°C.
[0081] Experimental procedure
[0082] Take about 25 mg of the crude drug of canagliflozin, accurately weigh it, put it in a 50 ml volumetric flask, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com