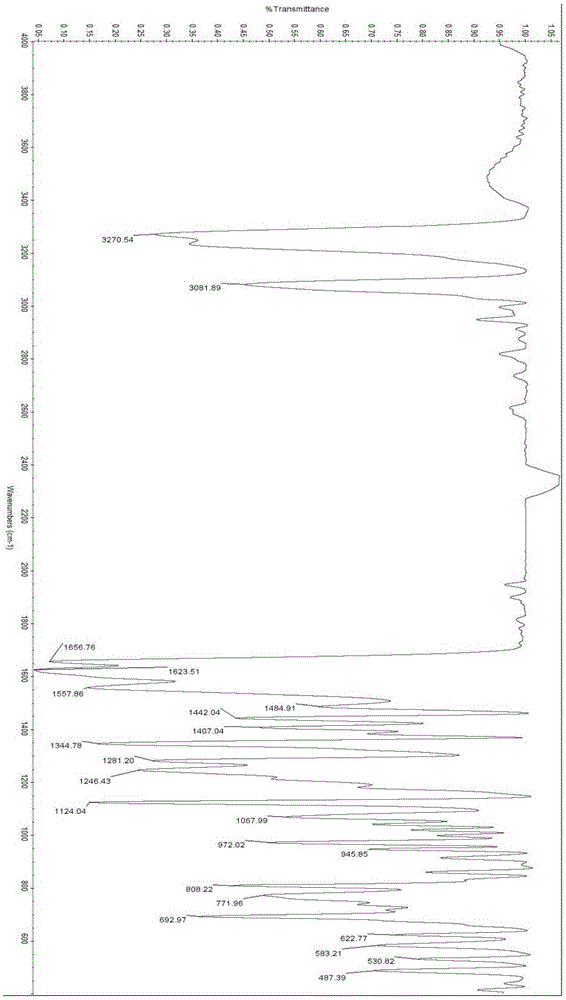
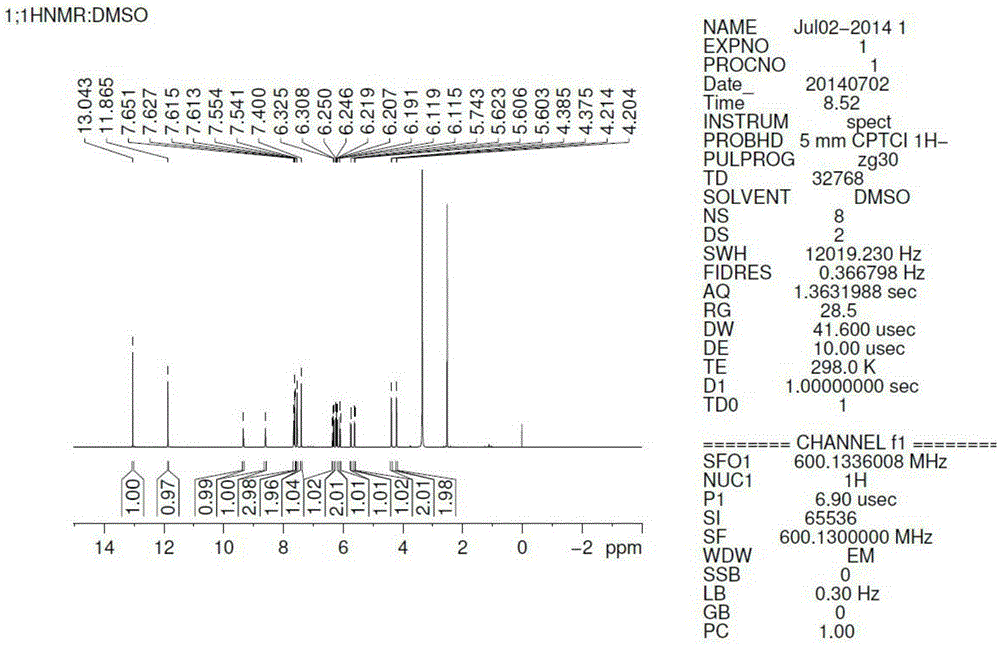
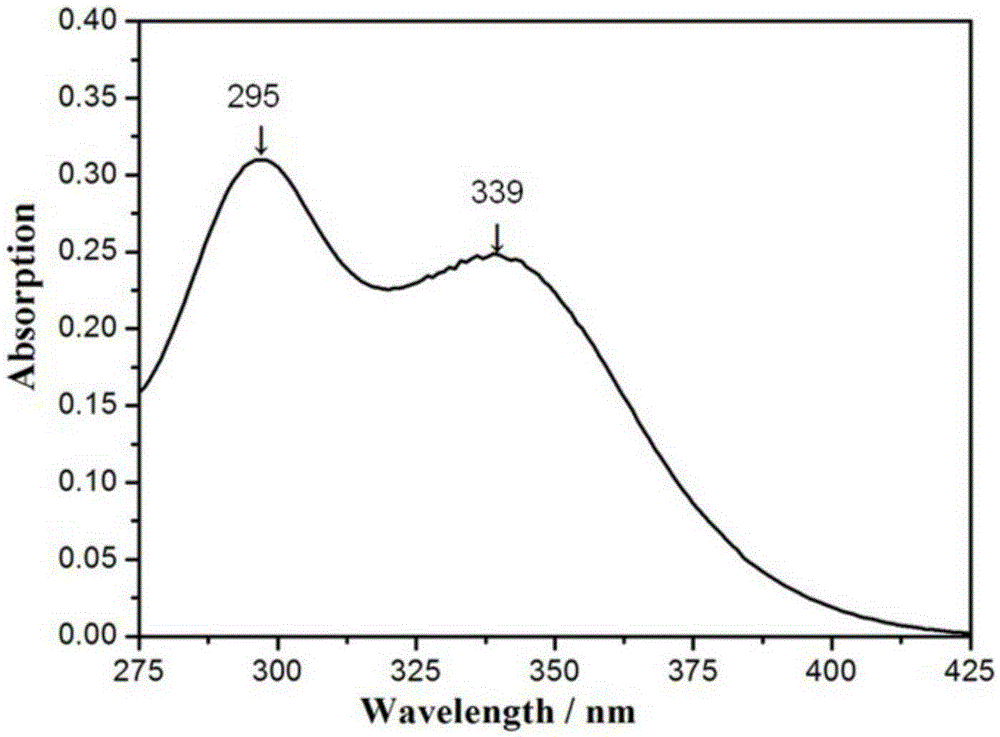
Reaction type ultraviolet light absorber as well as a preparation method and application thereof
A kind of ultraviolet, reactive technology, applied in the application of ultraviolet absorber in resin synthesis, reactive ultraviolet absorber, the preparation field of ultraviolet absorber
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1: Preparation has the resin of ultraviolet absorbing function
[0090] The implementation steps of this embodiment are as follows:
[0091] A. Friedel-Crafts alkylation reaction
[0092] In an organic solvent of 1.5 times of absolute ethanol based on the weight of the reactant, in the presence of a 2% concentrated sulfuric acid catalyst based on the weight of the reactant, 2,4-dihydroxybenzophenone and N-methylolacrylamide The reactants were subjected to Friedel-Crafts alkylation reaction at a temperature of 44°C for 4 days according to a mass ratio of 1:1.0, and then centrifuged at a speed of 800 rpm to obtain a precipitate;
[0093] B. Recrystallization
[0094] The precipitate obtained in step A is washed with distilled water to neutrality, and then the washed precipitate is recrystallized at room temperature for 0.1h in ethanol with a concentration of 42% by volume, and then centrifuged at 1000rpm at a rotating speed. The weight ratio of distilled wat...
Embodiment 2
[0099] Embodiment 2: Preparation has the resin of ultraviolet absorbing function
[0100] The implementation steps of this embodiment are as follows:
[0101] A. Friedel-Crafts alkylation reaction
[0102] In the acetone organic solvent of 1.3 times by reactant weight, in the presence of anhydrous aluminum trichloride catalyst of 8% by reactant weight, 2,4-dihydroxybenzophenone and N-methylol The acrylamide reactant was subjected to Friedel-Crafts alkylation reaction at a temperature of 28°C for 5 days according to a mass ratio of 1:0.9, and then centrifuged at a speed of 800 rpm to obtain a precipitate;
[0103] B. Recrystallization
[0104] The precipitate obtained in step A is washed with distilled water to neutrality, and then the washed precipitate is recrystallized at room temperature for 0.3h in ethanol with a concentration of 48% by volume, and then centrifuged at 1000rpm at a rotating speed. The weight ratio of distilled water to 1:4 was washed with distilled water...
Embodiment 3
[0109] Embodiment 3: Preparation has the resin of ultraviolet absorbing function
[0110] The implementation steps of this embodiment are as follows:
[0111] A. Friedel-Crafts alkylation reaction
[0112] In the isopropanol organic solvent of 1.4 times by reactant weight, in the presence of boron trifluoride catalyst of 1% by reactant weight, 2,4-dihydroxybenzophenone and N-methylol The acrylamide reactant was subjected to Friedel-Crafts alkylation reaction at a temperature of 10°C for 7 days according to a mass ratio of 1:0.8, and then centrifuged at a speed of 800 rpm to obtain a precipitate;
[0113] B. Recrystallization
[0114] The precipitate obtained in step A is washed with distilled water to neutrality, and then the washed precipitate is recrystallized at room temperature for 0.2h in ethanol with a concentration of 30% by volume, and then centrifuged at 1000rpm at a speed of rotation. The weight ratio to distilled water is 1:3, washed with distilled water for 4 ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com