Method for preparing tetraene methyl CAS
A technology of methyltetraene and hydroxyl, which is applied in the field of chemical preparation, can solve the problems of high cost of methyltetraene raw materials and difficulties in the 17-position hydroxyl of steroids, and achieve environmentally friendly costs, short process routes, and side effects. The effect of less product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
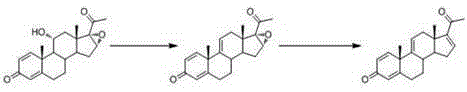
[0033] The first step is to add the dihydroxyprogesterone dehydrogenate to the dry 1000ml reaction flask 1 (10g, 0.029mol), 400mL of tetrahydrofuran, add phosphorus pentachloride (16g, 0.077mol) after cooling to -70℃, react at low temperature at -70℃ for 6 hours; after detecting no raw material by TLC , Add 10mL of water to quench the reaction, stir for 10 minutes; concentrate under reduced pressure, pour into ice water and stir for one hour, filter, wash with water, and dry at 60°C to obtain the elimination product 2 . The yield of this 11α hydroxyl elimination reaction is extremely high, the substrate conversion rate is above 95%, the molar yield is 95%, and the HPLC (HighPerformance Liquid Chromatography, high performance liquid chromatography) content is 95%.
[0034] The second step is to add the elimination product to the 2000ml reaction flask 2 (9g, 0.028mol), 400mL tetrahydrofuran, n-butyric acid (135g, 1.53mol), semicarbazide hydrochloride (1.08g, 9.68mmol) in 66 o C reac...
Embodiment 2
[0036] The first step is to add the dihydroxyprogesterone dehydrogenate to the dry 1000ml reaction flask 1 (10g, 0.029mol), 580mL of tetrahydrofuran, add phosphorus pentachloride (10g, 0.048mol) at -80℃, react at -80℃ for 10 hours; TLC (Thin Layer Chromatography) detects no raw material point, add 10mL of water The reaction was quenched and stirred for 10 minutes; concentrated under reduced pressure, poured into ice water and stirred for one hour, filtered, washed with water, and dried at 60°C to obtain the elimination product 2 , The molar yield is 92%, and the HPLC content is 90%.
[0037] The second step is to add the elimination product to the 2000ml reaction flask 2 (9g, 0.028mol), dichloromethane 400mL, n-butyric acid (135g, 1.53mol), semicarbazide hydrochloride (1.08g, 9.68mmol) in 50 o C react for 16 hours; after the reaction is over, cool to 0°C, add 20% sodium hydroxide solution to neutralize it, continue to stir for 1 hour, concentrate under reduced pressure, cool to 0°C...
Embodiment 3
[0039] The first step is to add the dihydroxyprogesterone dehydrogenate to the dry 1000ml reaction flask 1 (10g, 0.029mol), 290mL of tetrahydrofuran, add phosphorus pentachloride (30g, 0.144mol) at -50°C, react at low temperature at -50°C for 5 hours; TLC (Thin Layer Chromatography) detects no raw material point, add 30mL of water The reaction was quenched and stirred for 10 minutes; concentrated under reduced pressure, poured into ice water and stirred for one hour, filtered, washed with water, and dried at 60°C to obtain the elimination product 2 , The molar yield is 88%, and the HPLC content is 91%.
[0040] The second step is to add the elimination product to the 1000ml reaction flask 2 (9g, 0.028mol), 400mL tetrahydrofuran, acetic acid (135g, 2.25mol), semicarbazide hydrochloride (1.08g, 9.68mmol) in 66 o C react for 16 hours; concentrate under reduced pressure, add 180mL of water for precipitation, cool to 0℃, filter with suction, wash with water, and dry at 50℃ to obtain met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com