Method for preparing methyl tetraene
The technology of methyl tetraene and hydroxyl group is applied in the field of chemical preparation, which can solve the problems of difficulty in the 17-position hydroxyl group of steroids and high raw material cost of methyl tetraene, and achieves environmentally friendly cost, short process route, and improved selection. Effects of Sex and Yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
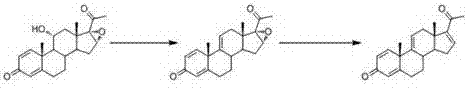
[0033] In the first step, add dihydroxyprogesterone dehydrogenate 1 (10g, 0.029mol) and 400mL tetrahydrofuran into a dry 1000ml reaction flask, add phosphorus pentachloride (16g, 0.077mol) after cooling down to -70°C, and React at low temperature at 70°C for 6 hours; after detecting no raw material point by thin layer chromatography (TLC), add 10 mL of water to quench the reaction, stir for 10 minutes; concentrate under reduced pressure, pour into ice water and stir for one hour, filter, wash with water, and store at 60°C Drying gave the eliminated product 2. The yield of this 11α hydroxyl elimination reaction is extremely high, the substrate conversion rate is above 95%, the molar yield is 95%, and the HPLC (High Performance Liquid Chromatography, high performance liquid chromatography) content is 95%.
[0034] In the second step, add elimination product 2 (9g, 0.028mol), tetrahydrofuran 400mL, n-butyric acid (135g, 1.53mol), semicarbazide hydrochloride (1.08g, 9.68 mmol) in ...
Embodiment 2
[0036] In the first step, add dihydroxyprogesterone dehydrogenate 1 (10g, 0.029mol) and 580mL tetrahydrofuran into a dry 1000ml reaction flask, add phosphorus pentachloride (10g, 0.048mol) at -80°C, and ℃ low temperature reaction for 10 hours; TLC (thin layer chromatography) to detect no raw material point, add 10 mL of water to quench the reaction, stir for 10 minutes; concentrate under reduced pressure, pour into ice water and stir for one hour, filter, wash with water, and dry at 60°C to obtain Product 2 was eliminated, the molar yield was 92%, and the HPLC content was 90%.
[0037] In the second step, add elimination product 2 (9g, 0.028mol), dichloromethane 400mL, n-butyric acid (135g, 1.53mol), semicarbazide hydrochloride (1.08g, 9.68 mmol) in 50 o C was reacted for 16 hours; after the reaction was completed, the temperature was lowered to 0°C, and 20% sodium hydroxide solution was added to neutralize to neutrality, and stirring was continued for 1 hour, concentrated und...
Embodiment 3
[0039] In the first step, add dihydroxyprogesterone dehydrogenate 1 (10g, 0.029mol) and 290mL tetrahydrofuran into a dry 1000ml reaction flask, add phosphorus pentachloride (30g, 0.144mol) at -50°C, and ℃ low temperature reaction for 5 hours; TLC (thin layer chromatography) detection of no raw material point, add 30mL of water to quench the reaction, stir for 10 minutes; concentrate under reduced pressure, pour into ice water and stir for one hour, filter, wash with water, dry at 60℃ to get Product 2 was eliminated, the molar yield was 88%, and the HPLC content was 91%.
[0040] In the second step, add elimination product 2 (9g, 0.028mol), tetrahydrofuran 400mL, acetic acid (135g, 2.25mol), semicarbazide hydrochloride (1.08g, 9.68 mmol) in 66 o C reaction for 16 hours; concentrated under reduced pressure, added 180 mL of water for water analysis, cooled to 0°C, filtered with suction, washed with water, and dried at 50°C to obtain methyltetraene 3 with a molar yield of 90% and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com