A kind of method utilizing cyclic reaction to prepare hexafluoroethane
A hexafluoroethane, cycle reaction technology, applied in the direction of halogen addition preparation, organic chemistry, etc., can solve the problems of detonation reaction, high risk of operation, slow reaction speed, etc., achieve mild reaction conditions, avoid cracking reaction, fast response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
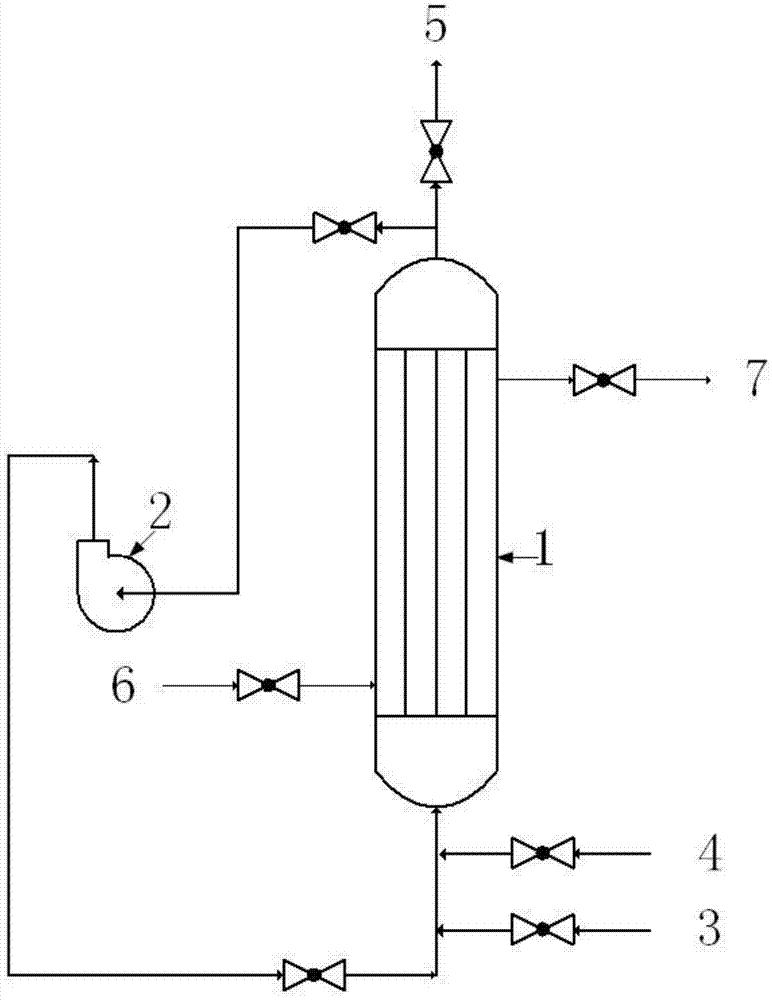
[0035] The volume of the tube-and-tube reactor 1 is 100L; the circulating air volume generated by the centrifugal fan 2 is 990L / min; the frozen brine with a mass fraction of 30% enters from the cooling medium inlet 6 and exits from the cooling medium outlet 7.
[0036] Fluorine gas with a flow rate of 4.95 L / min enters the tube-and-tube reactor 1 with a temperature of 10°C and a pressure of 0.15 MPa from the feed port 3 and tetrafluoroethylene with a flow rate of 4.95 L / min from the feed port 4. The space velocity of the gas in the tube-and-tube reactor 1 is 10 / min, and the reaction is carried out. Part of the reaction product obtained is circulated back to the column through the external circulation equipment centrifugal fan 2 of the tube-and-tube reactor 1 at the outlet of the tube-and-tube reactor 1. The inlet end of the tubular reactor 1 is mixed with the fluorine gas and tetrafluoroethylene that come in through the feed port 3 and the feed port 4, and then enters the tubul...
Embodiment 2
[0041] The volume of the tubular reactor 1 is 100L; the circulating air volume generated by the centrifugal fan 2 is 9.1L / min; water enters from the cooling medium inlet 6 and comes out from the cooling medium outlet 7; in the mixed gas of nitrogen and fluorine, fluorine The volume fraction of nitrogen is 10%, the volume fraction of nitrogen is 90%, and the flow rate of fluorine is 0.91L / min.
[0042] The mixed gas of nitrogen and fluorine enters the tube-and-tube reactor 1 with a temperature of 60°C and a pressure of 0.1 MPa from the feed port 3 and tetrafluoroethylene with a flow rate of 0.87 L / min from the feed port 4. The space velocity in the tube-and-tube reactor 1 is 0.2 / min, and the reaction is carried out. Part of the reaction product obtained is circulated back to the tube-and-tube reactor 1 through the centrifugal fan 2 of the external circulation equipment at the outlet of the tube-and-tube reactor 1. The inlet end of the reactor 1 is mixed with the mixed gas of ni...
Embodiment 3
[0047] The volume of the tube-and-tube reactor 1 is 100L; the circulating air volume generated by the centrifugal fan 2 is 289L / min; water enters from the cooling medium inlet 6 and comes out from the cooling medium outlet 7; in the mixed gas of nitrogen and fluorine, fluorine The volume fraction is 50%, the volume fraction of nitrogen gas is 50%, and the flow rate of fluorine gas is 3.6 L / min.
[0048]The mixed gas of nitrogen and fluorine enters the tube-and-tube reactor 1 with a temperature of 30°C and a pressure of 0.12 MPa from the feed port 3 and tetrafluoroethylene with a flow rate of 3.5 L / min from the feed port 4. The space velocity in the tube-and-tube reactor 1 is 3 / min, and the reaction is carried out. Part of the reaction product obtained is circulated back to the tube-and-tube reactor 1 through the external circulation equipment centrifugal fan 2 at the outlet of the tube-and-tube reactor 1. The inlet end of the reactor 1 is mixed with the mixed gas of nitrogen a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com