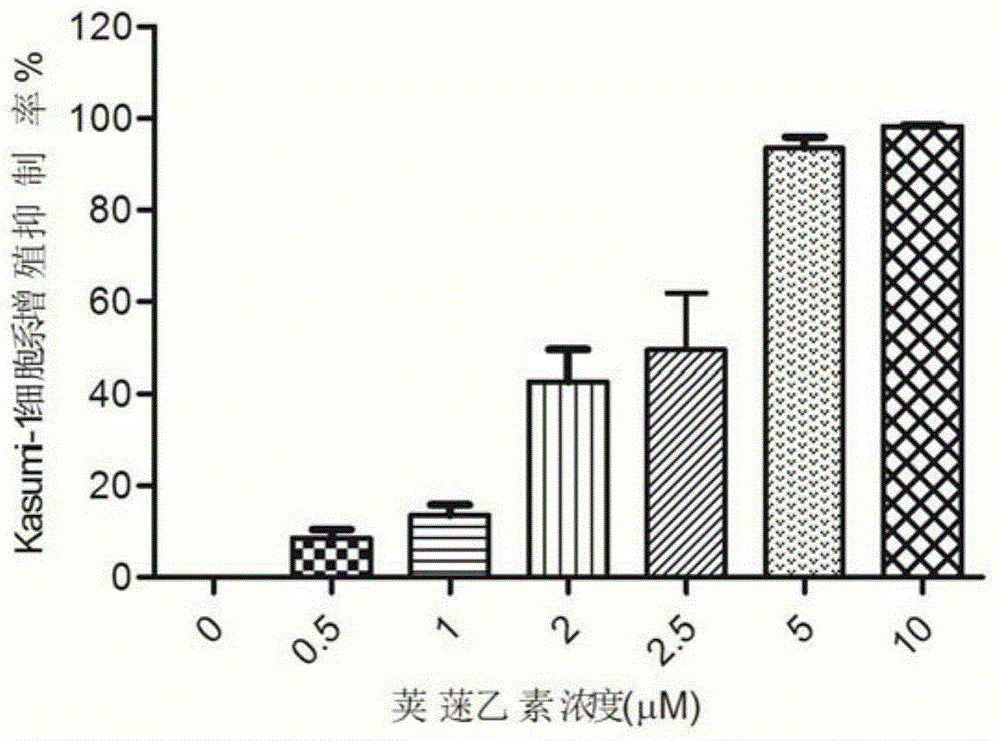
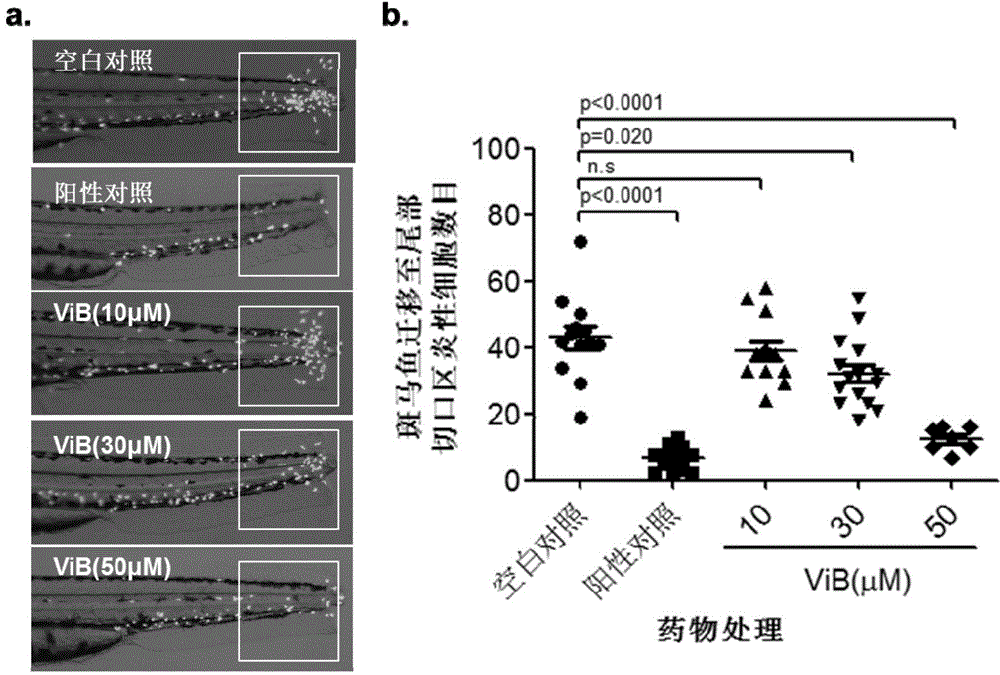
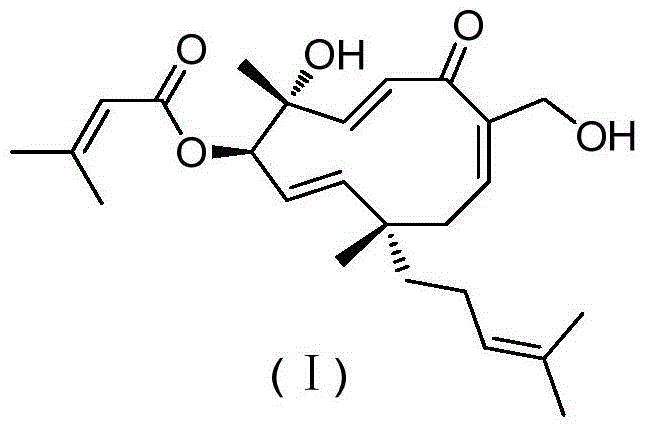
Vibsanin B, preparation method and uses thereof
A technology of viburnin and acetone is applied in the field of its preparation and viburnin, which can solve the problems that there is no anti-inflammatory activity or antitumor activity of viburnin and its mechanism of action.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0030] The preparation of viburnum B: after 22 kg of dry grass branches and leaves are pulverized, extract 3 times (2 days / time) with 80% acetone at room temperature, combine the extracts, concentrate under reduced pressure, add water to disperse, extract with ethyl acetate, water The volume ratio to ethyl acetate was 1:1.2, and the combined organic phase was concentrated to obtain 700 g of the crude extract. After the crude extract was dissolved, the sample was mixed with 100-200 mesh silica gel (the mass ratio of crude extract to silica gel was 1:1), Put on a 100-200 mesh silica gel column, and use petroleum ether / acetone system (volume ratio v / v is 9:1, 8:2, 7:3, 6:4, 0:1 in sequence) for gradient elution. Then the 7:3 and 6:4 section concentrates are mixed with polyamide respectively (the mass ratio of concentrate to polyamide is 1:1), and put on the RP-18 medium pressure chromatographic column (MPLC,), and the mass fraction is 75 %, 80%, 85% methanol aqueous solution elut...
preparation Embodiment 1
[0043] According to the method of Preparation Example 1, vibinin and salts made from organic acids (tartaric acid, citric acid, formic acid, oxalic acid, etc.) or inorganic acids (hydrochloric acid, sulfuric acid, phosphoric acid, etc.) were prepared, and conventionally added Water for injection, finely filtered, potted and sterilized to make injection solution.
preparation Embodiment 2
[0045] According to the method of Preparation Example 1, vibinin and salts made from organic acids (tartaric acid, citric acid, formic acid, oxalic acid, etc.) or inorganic acids (hydrochloric acid, sulfuric acid, phosphoric acid, etc.) were prepared, and dissolved In sterile water for injection, stir to dissolve, filter with a sterile suction filter funnel, and then aseptic fine filter, then pack in 2 ampoules, lyophilize at low temperature and seal aseptically to obtain a powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com