Method for synthesizing salazosulfapyridine using pyridazol as raw material
A technology of sulfasalazine and sulfasalazine, which is applied in the field of sulfasalazine, can solve the problems of less sources, unsatisfactory product purity, and difficult synthesis, and achieve the effects of high side reactions, large industrial production value, and guaranteed yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
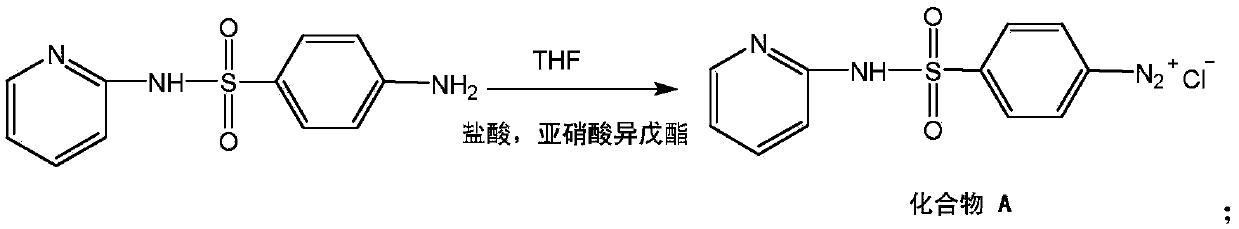
[0024] Sulfapyridine and the hydrochloric acid that substance quantity is 1.5 (taking the substance quantity of sulfapyridine as 1) fully mix, form A reaction liquid; Fully mix with THF with a mass of 5 (the mass of sulfapyridine is 1) to form a B reaction solution. The A reaction solution and the B reaction solution were transported to the tubular reactor through a peristaltic pump at a feed rate of 42 mL / min, mixed through a Y-shaped tube, and then entered into the tubular reactor (the temperature in which was controlled at 0°C). After staying for 40s, the material is discharged. The diazonium salt intermediate was isolated using known methods and this was named A.
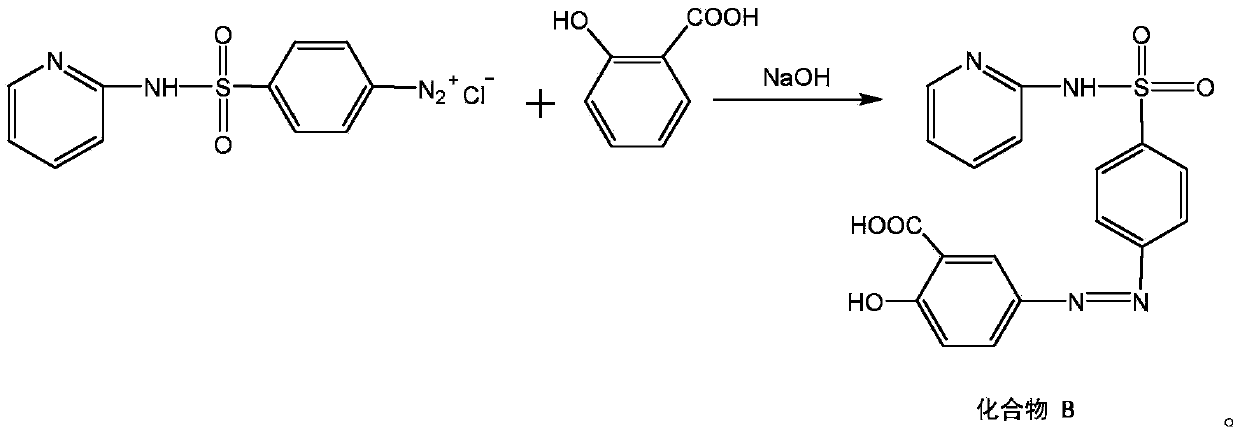
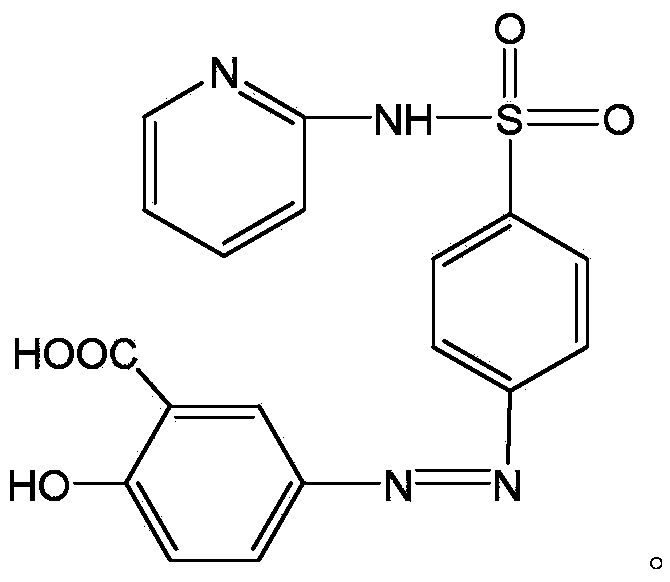
[0025] Put salicylic acid and partial sodium hydroxide (taking the amount of sulfapyridine as 1) in the reaction bottle. After the temperature was controlled to 5°C, the above-mentioned A was slowly started to be dropped into the above-mentioned reaction flask, and at the same time, the remaining sodium hydrox...
Embodiment 2
[0027] Sulfapyridine and hydrochloric acid with a substance quantity of 3 (calculated as 1 in the substance quantity of sulfapyridine) are fully mixed to form A reaction solution; Fully mix with THF with a mass of 10 (the mass of sulfapyridine is 1) to form a B reaction solution. The A reaction solution and the B reaction solution were transported to the tubular reactor through a peristaltic pump at a feed rate of 55 mL / min, mixed through a Y-shaped tube, and then entered into the tubular reactor (the temperature in which was controlled at 5°C). After staying for 15s, discharge the material. The diazonium salt intermediate was isolated using known methods and this was named A.
[0028] Put salicylic acid and partial sodium hydroxide (taking the amount of sulfapyridine as 1) in the reaction bottle. After the temperature was controlled to 10°C, the above-mentioned A was slowly started to be dropped into the above-mentioned reaction flask, and at the same time, the remaining so...
Embodiment 3
[0030] Sulfapyridine and the hydrochloric acid that substance quantity is 2.2 (taking the substance quantity of sulfapyridine as 1) fully mix, form A reaction liquid; Fully mix with THF with a mass of 7 (the mass of sulfapyridine is 1) to form a B reaction solution. The A reaction solution and the B reaction solution were transported to the tubular reactor through a peristaltic pump at a feed rate of 42 mL / min, mixed through a Y-shaped tube, and then entered into the tubular reactor (the temperature in which was controlled at 3°C). After staying for 40s, the material is discharged. The diazonium salt intermediate was isolated using known methods and this was named A.
[0031] Put salicylic acid and partial sodium hydroxide (taking the amount of sulfapyridine as 1) in the reaction bottle. After the temperature was controlled to 7°C, the above-mentioned A was slowly started to be dropped into the above-mentioned reaction flask, and at the same time, the remaining sodium hydrox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com