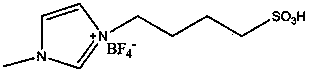Preparation method of poly epsilon-caprolactone
A technology of caprolactone and catalyst is applied in the field of preparing polyε-caprolactone, which can solve the problems of difficult removal of metal residues and inability to apply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] In a 1-liter glass bottle, add 300 grams of ε-caprolactone, 2 grams of ethylene glycol, and after three replacements with high-purity nitrogen, add 0.05 grams of 1-(4-sulfonic acid)butyl-3- Methylimidazole tetrafluoroborate, heated to 120-140 degrees, reacted for 12 hours, cooled to room temperature, dissolved the product with 2000 ml of chloroform, and precipitated with 5000 ml of ethanol to obtain 280 g of polyε-caprolactone. The weight average was determined by GPC The molecular weight is 30,000, and the molecular weight distribution is 1.3.
Embodiment 2
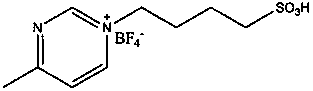
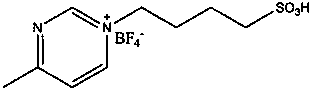
[0019] Add 15 kg of ε-caprolactone to a 20-liter stainless steel reactor with stirring, add 0.15 kg of butanediol, fill with nitrogen and replace the air three times, add 1-(4-sulfonic acid) butyl-3 under nitrogen protection - 75 grams of methylpyrazine tetrafluoroborate, polymerized at 130-150 degrees for 12 hours, then vacuum (vacuum degree 1-10mmHg) at this temperature to remove unreacted monomers to obtain 14.5 kilograms of polyε-hexane Lactone, measured by GPC, has a weight average molecular weight of 10,000 and a molecular weight distribution of 1.2.
Embodiment 3
[0021] Add 15 kg of ε-caprolactone and 0.75 kg of 1,2-propanediol to a 20-liter stainless steel reactor with stirring, fill with nitrogen and replace the air three times, and add 1-(4-sulfonic acid) butyl- 15 grams of 3-methylpyrazine tetrafluoroborate, polymerized at 90-120 degrees for 12 hours, then vacuum (vacuum degree 1-10mmHg) at this temperature to remove unreacted monomers and impurities to obtain 14.8 kilograms of polyε -Caprolactone, the weight average molecular weight measured by GPC is 2500, and the molecular weight distribution is 1.24.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com