Prothrombin time assay kit and preparation method thereof
A prothrombin time and kit technology, applied in biochemical equipment and methods, microbial measurement/inspection, etc., can solve problems such as cumbersome operation process, inaccurate test results, and increased patient burden, and achieve quick and easy operation, Avoid large variances, avoid effects of bottle-to-bottle variance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
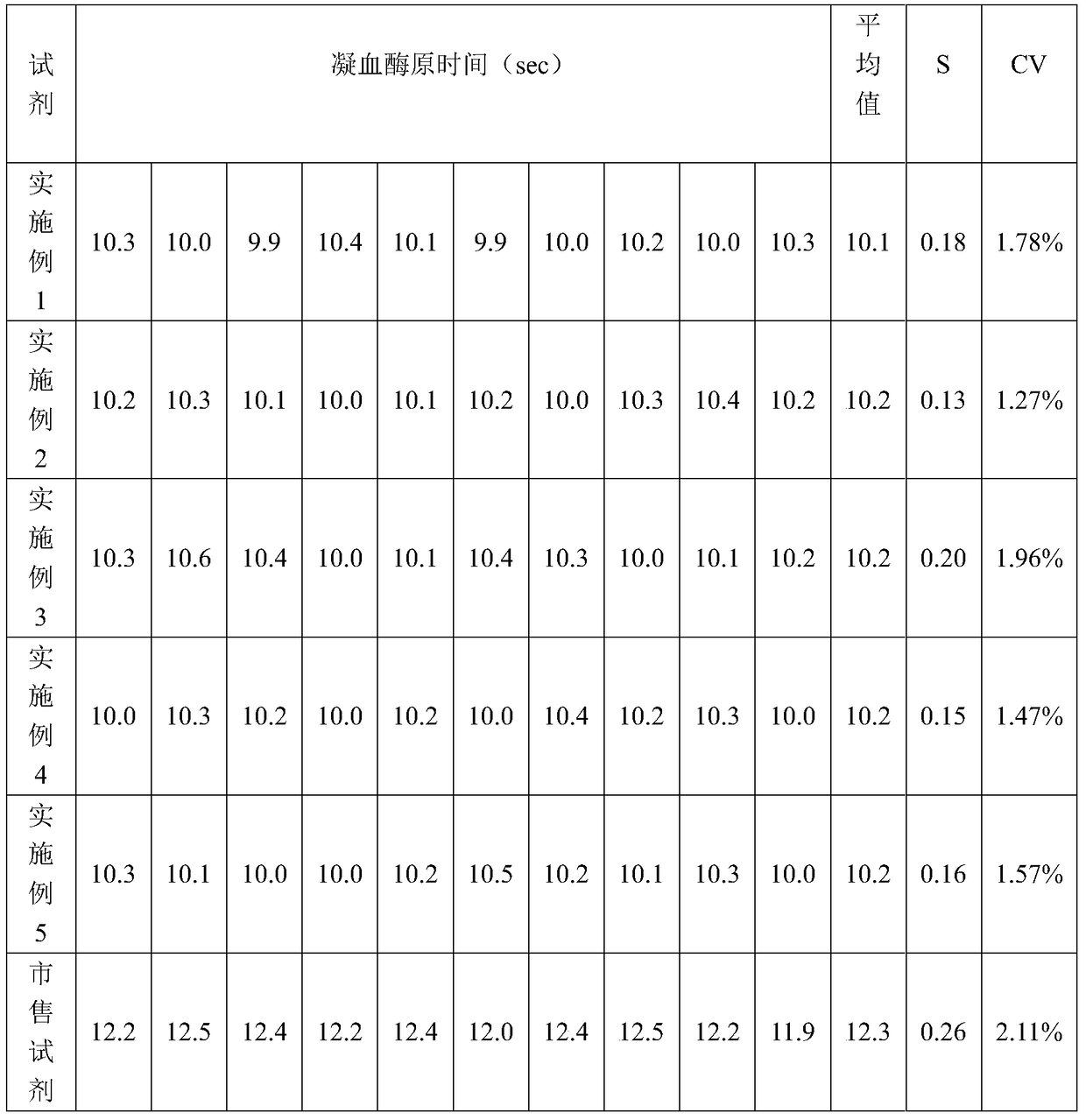
[0021] The prothrombin time assay kit includes a prothrombin time assay reagent, the prothrombin time assay reagent is composed of a buffer system and thromboplastin dissolved in the buffer system, the thromboplastin is a tissue factor ester, The amount of tissue factor esterified product in the buffer system accounts for 0.05wt% of the buffer weight; the contents of each component of the buffer system are: 3wt% bovine serum albumin, 20mM calcium chloride, 20mM HEPES, 0.5wt% sodium azide, 0.05wt% sodium chloride, and the rest are water, and the pH value of the buffer solution system is 7.0.
[0022] The method for preparing the prothrombin time assay kit comprises the following steps: (1) preparing a buffer system according to different proportions, adjusting the pH to 7.0, adding tissue factor esterified product, stirring and mixing to obtain the initial prothrombin time Determination of reagents; (2) Perform quality control measurements on the initial reagents on the hemaggl...
Embodiment 2
[0025] The prothrombin time assay kit comprises a prothrombin time assay reagent, the prothrombin time assay reagent is composed of a buffer system and thromboplastin dissolved in the buffer system, and the thromboplastin is tissue factor ester Compound, the amount of tissue factor esterified product in the buffer system accounted for 0.3wt% of the buffer weight; the contents of each component of the buffer system: 1wt% bovine serum albumin, 9mM calcium chloride, 45mM buffer, 0.1wt% sodium azide, 0.3wt% sodium chloride, and water as the rest, and the pH value of the buffer system is 5.0; wherein, the buffering agent is a mixture of MPOS and PBS with a volume ratio of 1:2.5.
[0026] The preparation method of the prothrombin time assay kit is the same as in Example 1.
[0027] Apply the test kit obtained in this embodiment to measure the prothrombin time of the plasma to be tested on a hemagglutination analyzer, the measurement method is the same as in Example 1, do ten paralle...
Embodiment 3
[0029] The prothrombin time assay kit comprises a prothrombin time assay reagent, the prothrombin time assay reagent is composed of a buffer system and thromboplastin dissolved in the buffer system, and the thromboplastin is tissue factor ester Compound, the amount of tissue factor esterified product in buffer system accounts for 1wt% of buffer weight; The content of each component of buffer system is: the bovine serum albumin of 0.5wt%, the calcium chloride of 6mM, the TRICINE of 200mM, 0.05 Wt% sodium azide, 1wt% sodium chloride, and the rest are water, and the pH value of the buffer solution system is 6.0.
[0030] The preparation method of the prothrombin time assay kit is the same as in Example 1.
[0031] Apply the test kit obtained in this embodiment to measure the prothrombin time of the plasma to be tested on a hemagglutination analyzer, the measurement method is the same as in Example 1, do ten parallel experiments, record the data, and analyze the repeatability of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com