A kit for measuring activated partial thromboplastin time (aptt)
A technology of thromboplastin time and thromboplastin is applied in the field of kits for determining activated partial thromboplastin time (APTT) in blood, which can solve the problems of large differences in measurement results and unstable quality of kits, and avoid bottle Differences between, avoid large differences, and ensure the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of kit for measuring activated partial thromboplastin time (APTT)
[0038] formula:
[0039] R1 reagent: 0.1mM ellagic acid, 0.25% rabbit cephalin, 3% dextran 20, 0.5% Tween20, 1% bovine serum albumin, 20mM pH 7.6 Tris-HCl buffer, 0.02% ProClin 300.
[0040] R2 reagent: 25mM calcium chloride, 0.01% thimerosal.
[0041] Preparation:
[0042] (1) Weigh rabbit cephalin, dextran 20, Tween20, bovine serum albumin, ProClin 300 and dissolve in 20mMpH 7.6 Tris-HCl buffer solution, stir well and add ellagic acid to the required concentration to prepare R1 reagent;
[0043] (2) Weigh calcium chloride and thimerosal respectively and add them into sterile water to prepare R2 reagent.
Embodiment 2
[0044] Example 2 Detection of activated partial thromboplastin time
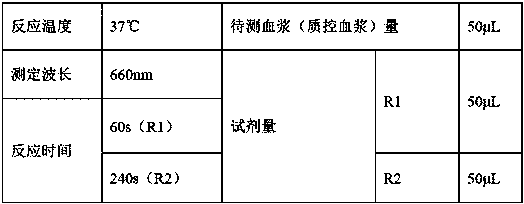
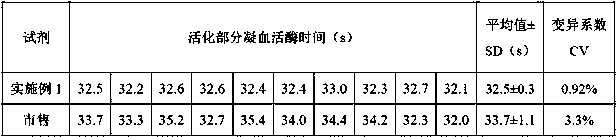
[0045] Adopt the kit of embodiment 1 and commercially available reagent to measure the same plasma sample with full-automatic hemagglutination instrument, the main parameter settings of the instrument are shown in Table 1; record the activated partial thromboplastin time, the repeatability of reagent of the present invention and commercially available reagent The comparison results are shown in Table 2. As can be seen from the data in the table, compared with commercially available reagents, the reagent of the present invention has a smaller coefficient of variation and better repeatability.
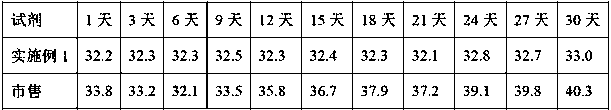
[0046] Open the bottle of the reagent of the present invention and the commercially available reagent at the same time, the commercially available reagent is used after reconstitution of freeze-dried powder, the reagent of the present invention is used directly, and the quality control test is performed on the same coa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com