Activated partial thromboplastin time assay kit and preparation method thereof
A technology of thromboplastin time and reagent kit, which is applied in the biological field, can solve the problems of waste, reagent stability and measurement effect deterioration, and achieve the effects of saving dosage, avoiding differences between bottles, and avoiding large differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
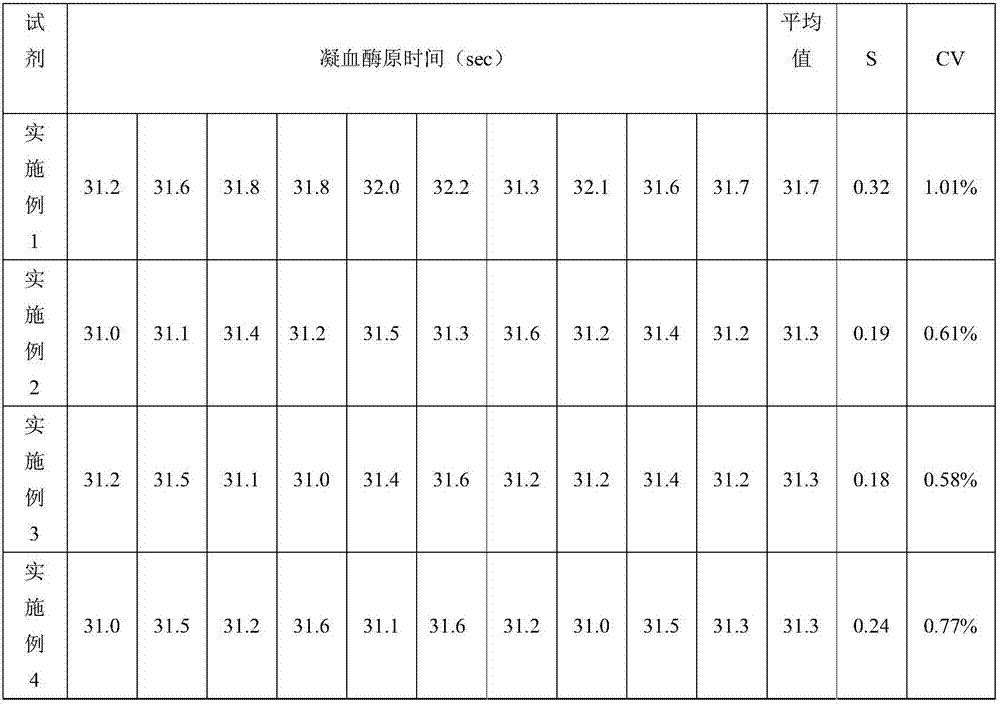
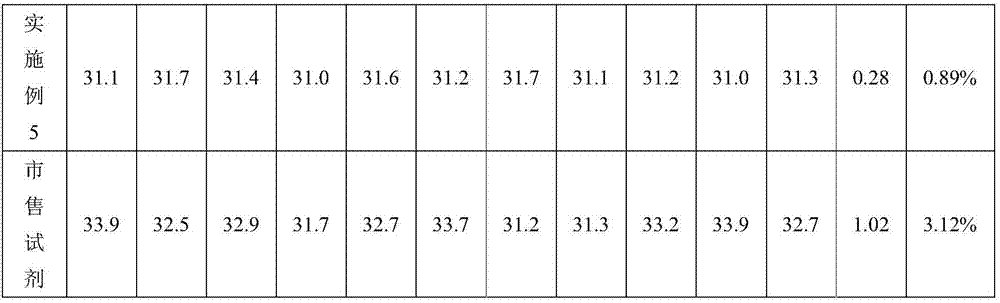
[0025] Activated partial thromboplastin time determination kit, including phospholipid reagent and calcium chloride reagent, the two reagents are stored in different containers; the phospholipid reagent is composed of ellagic acid, phospholipid and buffer system, the content of ellagic acid is 2g / L , the content of phospholipids is 2.5wt%, and the content of each component in the buffer system is: Tris-Hcl of 1M, Kathon of 0.01wt%, polyethylene glycol of 1.0wt%, the rest are water, and the pH of the buffer system is 6.0; The working concentration of calcium chloride reagent is 15 mM. When using the kit of the present invention, the volume ratio of the plasma to be tested and the amount of phospholipid reagent is 1:0.85-1.1.
[0026] The method for preparing the above-mentioned activated partial thromboplastin time assay kit comprises the following steps: (1) preparing a buffer system, adjusting the pH to 6.0-8.0, adding a solid activator and mixing to obtain an initial mixed s...
Embodiment 2
[0029]Activated partial thromboplastin time assay kit, including phospholipid reagent and calcium chloride reagent, the two reagents are stored in different containers; the phospholipid reagent is composed of bentonite, rabbit cephalin and buffer system, and the content of bentonite is 0.05 g / L, the content of rabbit cephalin is 7wt%, and the content of each component in the buffer system is: the MPOS of 0.4M, the sodium azide of 0.1wt%, the polyethylene glycol of 0.8wt%, all the other are water; The pH of the system is 7.8, and the working concentration of calcium chloride reagent is 20mM.
[0030] The preparation method of the activated partial thromboplastin time assay kit is the same as in Example 1.
[0031] Apply the test kit obtained in this embodiment to measure the prothrombin time of the plasma to be tested on a semi-automatic hemagglutination instrument, the measurement method is the same as in Example 1, do ten parallel experiments, record the data, and analyze the...
Embodiment 3
[0033] Activated partial thromboplastin time determination kit, including phospholipid reagent and calcium chloride reagent, the two reagents are stored in different containers; phospholipid reagent is composed of solid activator, rabbit cephalin and buffer system, the content of solid activator is 1.0 g / L, the content of rabbit cephalin is 5wt%, and the content of each component in the buffer system is: the Hepes of 0.75M, the stabilizing agent of 0.05wt%, the polyethylene glycol of 0.3wt%, all the other are water; The pH of the solution is 6.0, and the working concentration of calcium chloride reagent is 35mM; wherein, the solid activator is a mixture of ellagic acid, kaolin and magnesium aluminum silicate with a quality of 1:0.5:2, and the stabilizer is Kathon, glycine, serum white 1:0.86:1.1 mixture of egg whites.
[0034] The preparation method of the activated partial thromboplastin time assay kit is the same as in Example 1.
[0035] Apply the test kit obtained in this...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com