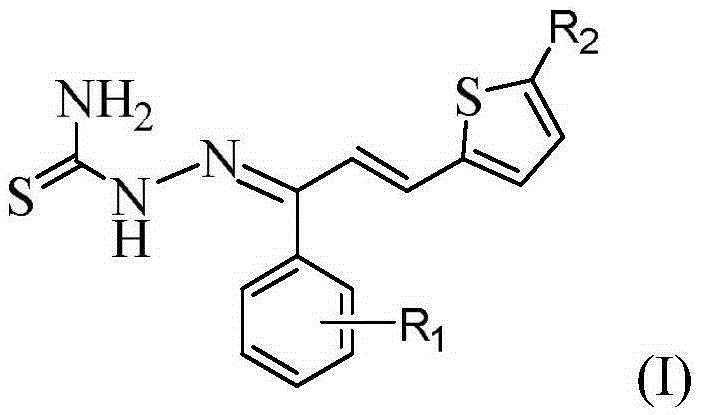
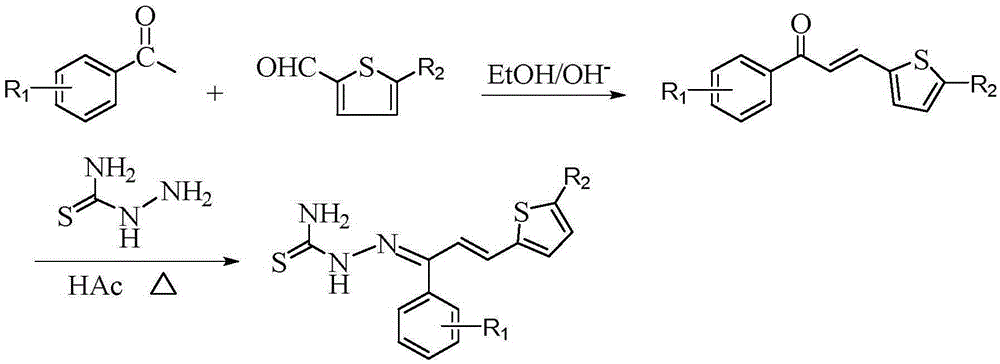
Thiosemicarbazone derivatives, preparation method and applications thereof
A technology of thiosemicarbazone and its derivatives, which is applied in the field of pesticides to achieve good control effect, simple structure and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] compound (C 14 h 12 N 3 S 2 Br) preparation
[0034] (1) Synthesis of intermediate 1-(4-bromophenyl)-3-(2-thienyl)-2-propen-1-one
[0035] Dissolve 0.02 mol of 4-bromoacetophenone in 30 mL of absolute ethanol, and then add 10 mL of 10% NaOH solution thereto. Under ice-bath stirring, the mixture of 0.02mol 2-thiophenecarbaldehyde and 20mL of absolute ethanol was slowly dropped into the above-mentioned mixed solution with a constant pressure dropping funnel, reacted at 0-5°C, and was tested with a thin-layer silica gel plate (TLC ) to check that the reaction is complete. After the reaction is completed, add 3-4 times the volume of distilled water to the mixture, and adjust its pH value to neutral with 10% HCl, there is a precipitate, filter, wash, and recrystallize with absolute ethanol to obtain intermediate 1 -(4-bromophenyl)-3-(2-thienyl)-2-propen-1-one.
[0036] (2) Synthesis of the target compound
[0037] Dissolve 0.015 mol of thiosemicarbazide in 20 mL of...
Embodiment 2
[0040] compound (C 14 h 12 N 3 S 2 Cl) Preparation
[0041] (1) Synthesis of intermediate 1-(3-chlorophenyl)-3-(2-thienyl)-2-propen-1-one
[0042] Dissolve 0.02 mol of 3-chloroacetophenone in 30 mL of absolute ethanol, and then add 10 mL of 10% NaOH solution thereto. Under ice-bath stirring, the mixture of 0.02mol 2-thiophenecarbaldehyde and 20mL of absolute ethanol was slowly dropped into the above-mentioned mixed solution with a constant pressure dropping funnel, reacted at 0-5°C, and was tested with a thin-layer silica gel plate (TLC ) to check that the reaction is complete. After the reaction is completed, add 3-4 times the volume of distilled water to the mixture, and adjust its pH value to neutral with 10% HCl, there is a precipitate, filter, wash, and recrystallize with absolute ethanol to obtain intermediate 1 -(3-Chlorophenyl)-3-(2-thienyl)-2-propen-1-one.
[0043] (2) Synthesis of the target compound
[0044] Dissolve 0.015 mol of thiosemicarbazide in 20 mL...
Embodiment 3
[0047] compound (C 14 h 11 N 3 S 2 Preparation of FBr)
[0048] (1) Synthesis of intermediate 1-(4-fluorophenyl)-3-(5-bromo-2-thienyl)-2-propen-1-one
[0049] Dissolve 0.02 mol of 4-fluoroacetophenone in 30 mL of absolute ethanol, and then add 10 mL of 10% NaOH solution thereto. While stirring in an ice bath, slowly drop a mixture of 0.02mol 5-bromo-2-thiophenecarbaldehyde and 20mL of absolute ethanol into the above mixed solution with a constant pressure dropping funnel, react at 0-5°C, and use a thin layer Silica gel plates (TLC) were used to check the completion of the reaction. After the reaction is completed, add 3-4 times the volume of distilled water to the mixture, and adjust its pH value to neutral with 10% HCl, there is a precipitate, filter, wash, and recrystallize with absolute ethanol to obtain intermediate 1 -(4-fluorophenyl)-3-(5-bromo-2-thienyl)-2-propen-1-one.
[0050] (2) Synthesis of the target compound
[0051] Dissolve 0.015 mol of thiosemicarbaz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com