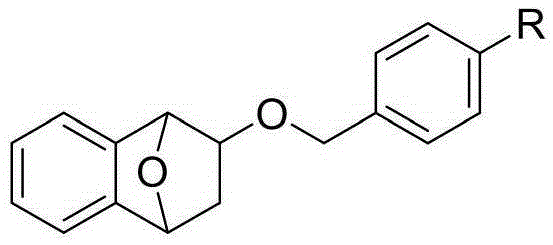
Novel compound as well as preparation method and application thereof
A compound and a new type of technology, applied in the field of new compounds and their preparation, can solve the problem of low enantioselectivity of hydrosilation products, achieve high yield, expand application, and inhibit the growth of weeds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
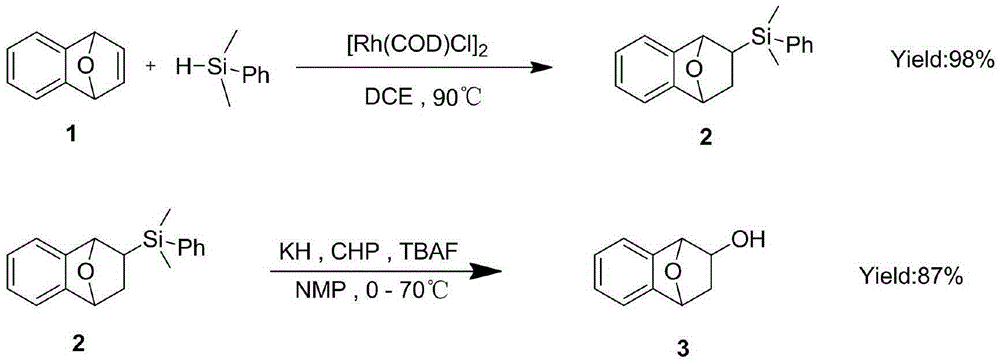
[0034] 1. In the glove box, put [Rh(COD)Cl] 2 (5mg, 0.01mmol) and 1 (129.6mg, 0.9mmol) were added to the reaction tube, and then 3mL of DCE was added, sealed and taken out. Use a syringe to add dimethylphenylsilane (690ul, 4.5mmol) to the reaction tube. Put it into an oil bath at a constant temperature of 90°C for 1 hour. After removing the solvent, petroleum ether / ethyl acetate 20:1 column chromatography gave 2, a white solid.
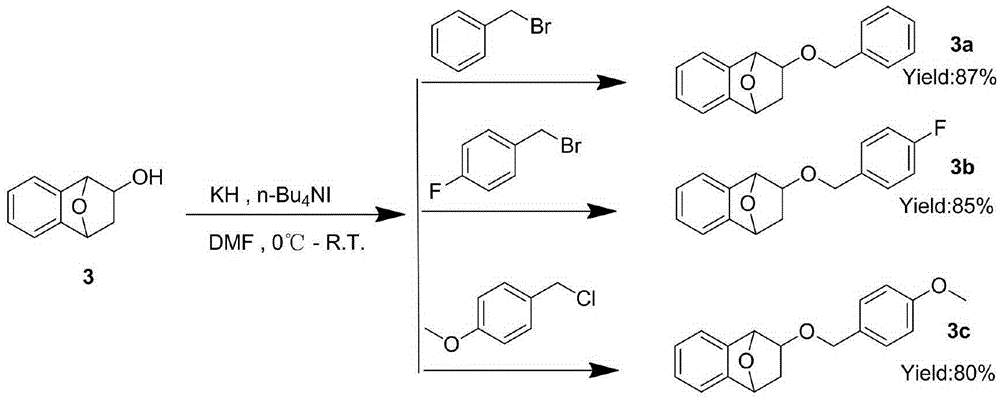
[0035] 2. Disperse 80 mg of KH (2 mmol) into 1 mL of NMP to make a suspension at 0°C. Add CHP (380 mg, 2 mmol) to the suspension, stir for 20 minutes, and then add dropwise to the reaction solution. 2 mL of NMP 2 (112 mg, 0.4 mmol) and TBAF (220 mg, 0.84 mg). After stirring for 10 minutes at room temperature, the temperature was raised to 70° C. and reacted for 5 hours. After cooling the reaction solution to room temperature, add 5 mL of saturated Na 2 S 2 O 3 The solution was stirred for 30 minutes. Extract with methyl tert-butyl ether (15 mL*5), co...
Embodiment 2
[0038] 1. In the glove box, put [Rh(COD)Cl] 2 (5mg, 0.01mmol) and 1 (129.6mg, 0.9mmol) were added to the reaction tube, and then 3mL of DCE was added, sealed and taken out. Use a syringe to add dimethylphenylsilane (690ul, 4.5mmol) to the reaction tube. Put it into an oil bath at a constant temperature of 90°C for 1 hour. After removing the solvent, petroleum ether / ethyl acetate 20:1 column chromatography gave 2, a white solid.
[0039] 2. Disperse 80 mg of KH (2 mmol) into 1 mL of NMP to make a suspension at 0°C. Add CHP (380 mg, 2 mmol) to the suspension, stir for 20 minutes, and then add dropwise to the reaction solution. 2 mL of NMP 2 (112 mg, 0.4 mmol) and TBAF (220 mg, 0.84 mg). After stirring for 10 minutes at room temperature, the temperature was raised to 70° C. and reacted for 5 hours. After cooling the reaction solution to room temperature, add 5 mL of saturated Na 2 S 2 O 3 The solution was stirred for 30 minutes. Extract with methyl tert-butyl ether (15 mL*5), co...
Embodiment 3
[0042] 1. In the glove box, put [Rh(COD)Cl] 2 (5mg, 0.01mmol) and 1 (129.6mg, 0.9mmol) were added to the reaction tube, and then 3mL of DCE was added, sealed and taken out. Use a syringe to add dimethylphenylsilane (690ul, 4.5mmol) to the reaction tube. Put it into an oil bath at a constant temperature of 90°C for 1 hour. After removing the solvent, petroleum ether / ethyl acetate 20:1 column chromatography gave 2, a white solid.
[0043] 2. Disperse 80 mg of KH (2 mmol) into 1 mL of NMP to make a suspension at 0°C. Add CHP (380 mg, 2 mmol) to the suspension, stir for 20 minutes, and then add dropwise to the reaction solution. 2 mL of NMP 2 (112 mg, 0.4 mmol) and TBAF (220 mg, 0.84 mg). After stirring for 10 minutes at room temperature, the temperature was raised to 70° C. and reacted for 5 hours. After cooling the reaction solution to room temperature, add 5 mL of saturated Na 2 S 2 O 3 The solution was stirred for 30 minutes. Extract with methyl tert-butyl ether (15 mL*5), co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com