Method for preparing aldehyde compound by olefin hydroformylation
A technology of aldehyde compounds and olefin hydroformyl, which is applied in the chemical industry, can solve the problems of high solvent toxicity, complicated process, and many high boiling substances, and achieve the effects of increasing solubility, stable catalytic system, and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
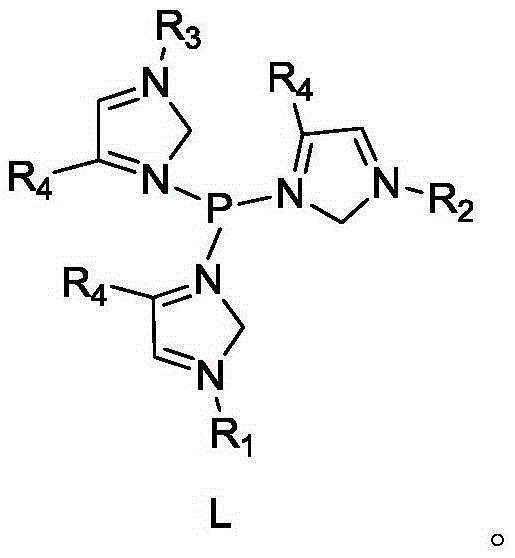
[0029] Phosphorus ligand L1 and its catalyst mother liquor Q 1 and Q 2 Preparation of:
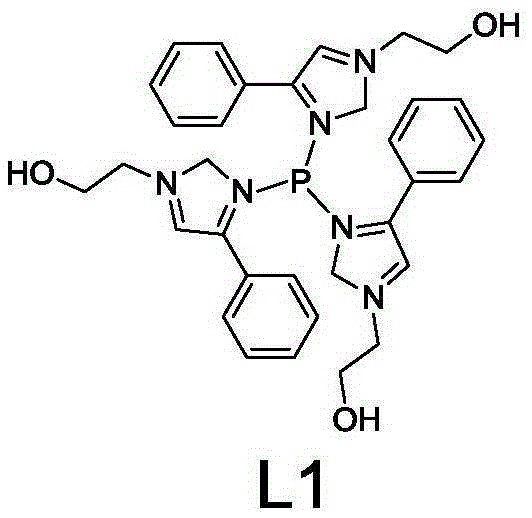
[0030] 43.3g phenylethylenediamine Mix well with 24.3g of 37% formaldehyde aqueous solution, react at 60°C for 70min, then add 13.7g of PCl dropwise to the system 3 With 24.8g 2-chloroethanol, add 100g deionized water at the same time, continue to stir for 50min, obtain the aqueous solution containing phosphorus ligand L1, the concentration is 36.6wt%, adopt NMR qualitative, 1 HNMR data (CDCl 3 as solvent, TMS as internal standard): 7.98(m, 3H, -CH), 7.52(m, 9H, -CH), 7.5(m, 3H, NCH-), 4.17(t, 6H, HOCH 2 -),3.65(s,3H,-HO),3.6(s,6H,N-CH 2 -N),1.68(t,6H,N-CH 2 -).
[0031] The structural formula of L1 is:
[0032]
[0033] Add 1.02g rhodium dicarbonyl acetylacetonate to the 100g aqueous solution containing L1 ligand prepared above, and stir for 30min under nitrogen protection to obtain catalyst mother liquor Q 1 .
[0034] 100gL of the above prepared 1 Add 0.6g rhodium dicarbo...
Embodiment 1
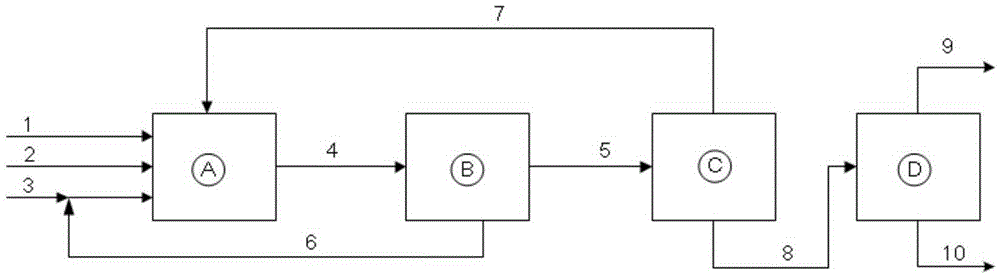
[0047] Catalyst mother liquor Q 1 ,Such as figure 1 As shown, the air in reactor A was replaced with nitrogen for 3 times before feeding, and the catalyst mother liquor 3 and olefin 1 were respectively added to reactor A by using an advection pump. The volume ratio of catalyst mother liquor 3 and olefin 3 was 0.5, stirred and mixed evenly, and then Replace with synthesis gas 2 for 3 times, then increase the pressure to 2.0-15MPa, raise the temperature to 80-150°C, and react at constant pressure for 3-5 hours. Cool down to room temperature, release the pressure, take out the reaction solution, and analyze the composition of the reaction solution by gas chromatography. The results show that the conversion rate of olefins is 50-65%, and the selectivity of aldehydes is >99.0%. The specific results are shown in Table 1. Reactor discharge 4 enters in phase separator B, is separated into oil phase 5 and water phase 6, and water phase 6 is circulated back to reactor A, and oil phase ...
Embodiment 2
[0051] Catalyst mother liquor Q 2 , The volume ratio of the catalyst mother liquid to the olefin is 1.0, pressurize to 1.8-14MPa, heat up to 85-165°C, and react at constant pressure for 3-5 hours, and the rest are the same as in Example 1. Cool down to room temperature, release the pressure, take out the reaction solution, and analyze the composition of the reaction solution by gas chromatography. The results show that the conversion rate of olefins is 53-69%, and the selectivity of aldehydes is >99.0%. The specific results are shown in Table 2.
[0052] Table 2:
[0053] Olefin
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com